Abstract
Prophenoloxidase (proPO), an enzyme that is the terminal component of the so-called proPO activating system, a defense and recognition system in crustaceans and insects, has been purified and cloned from a crayfish blood cell cDNA library. The deduced amino acid sequence codes for a polypeptide with a mass of 80,732 Da, which is close to 76 kDa, the apparent mass of the purified enzyme. proPO contains two copper atoms, and two putative copper-binding sites were found in the deduced amino acid sequence. Sequence comparisons show that these putative copper-binding sites are similar to the corresponding sites in arthropod hemocyanins and also, although the sequence similarities are less extensive, similar to tyrosinases from vertebrates and microorganisms. The purified enzyme is a typical tyrosinase because it hydroxylates monophenols and oxidizes o-diphenols but does not oxidize p-diphenols. If a homogeneous preparation of crayfish proPO were incubated with a homogeneous sample of the proPO activating enzyme, a serine proteinase, the cleavage of proPO by this trypsin-like enzyme was found to occur between Arg-176 and Thr-177.
Full text
PDF
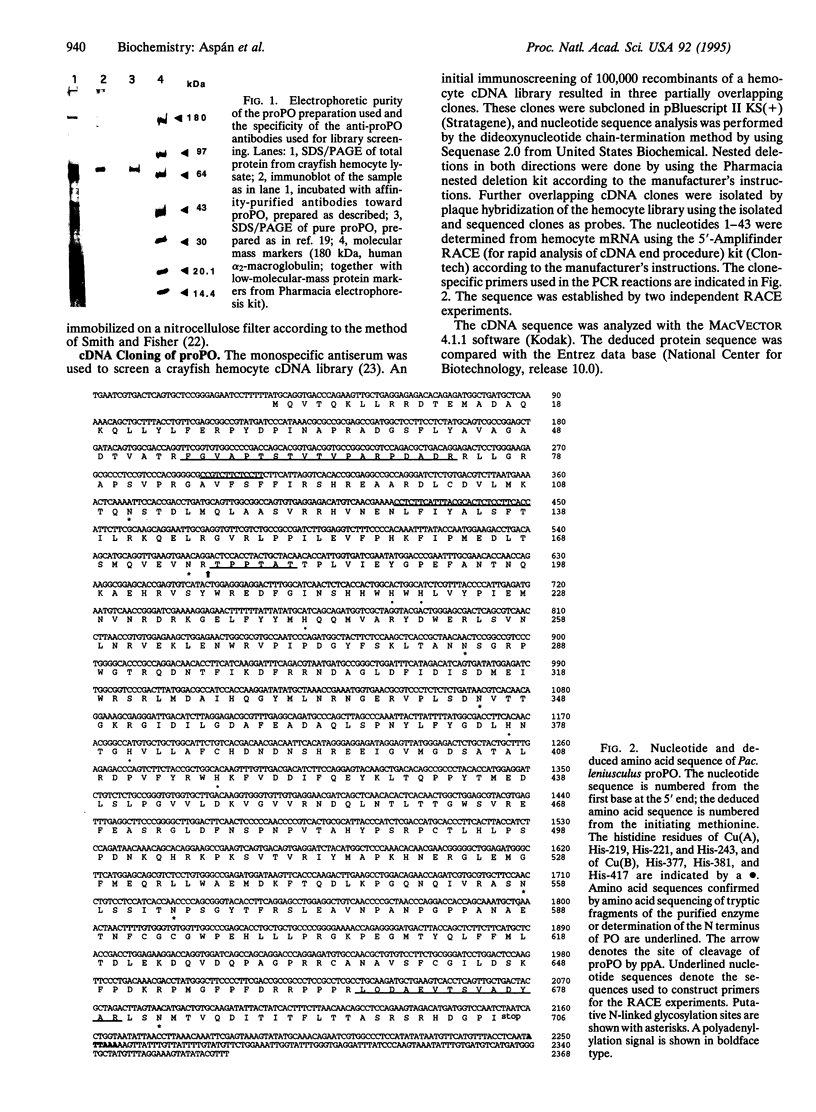

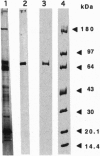
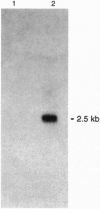
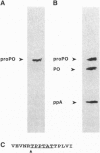
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida M. The prophenoloxidase cascade in insect immunity. Res Immunol. 1990 Nov-Dec;141(9):908–910. doi: 10.1016/0923-2494(90)90191-z. [DOI] [PubMed] [Google Scholar]
- Bernan V., Filpula D., Herber W., Bibb M., Katz E. The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterization of the gene product. Gene. 1985;37(1-3):101–110. doi: 10.1016/0378-1119(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L., Liang Z., Duvic B., Keyser P., Hellman U., Palva E. T., Iwanaga S., Söderhäll K. Structure and biological activity of a 1,3-beta-D-glucan-binding protein in crustacean blood. J Biol Chem. 1994 Nov 25;269(47):29462–29467. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins F. H., Besansky N. J. Vector biology and the control of malaria in Africa. Science. 1994 Jun 24;264(5167):1874–1875. doi: 10.1126/science.8009215. [DOI] [PubMed] [Google Scholar]
- Collins F. H., Sakai R. K., Vernick K. D., Paskewitz S., Seeley D. C., Miller L. H., Collins W. E., Campbell C. C., Gwadz R. W. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986 Oct 31;234(4776):607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- Duvic B., Söderhäll K. Purification and characterization of a beta-1,3-glucan binding protein from plasma of the crayfish Pacifastacus leniusculus. J Biol Chem. 1990 Jun 5;265(16):9327–9332. [PubMed] [Google Scholar]
- Duvic B., Söderhäll K. Purification and partial characterization of a beta-1,3-glucan-binding-protein membrane receptor from blood cells of the crayfish Pacifastacus leniusculus. Eur J Biochem. 1992 Jul 1;207(1):223–228. doi: 10.1111/j.1432-1033.1992.tb17041.x. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Strunk K. M., Spritz R. A. Organization and nucleotide sequences of the human tyrosinase gene and a truncated tyrosinase-related segment. Genomics. 1991 Mar;9(3):435–445. doi: 10.1016/0888-7543(91)90409-8. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jr, Ekel T. M., Montague P. M., Hearing E. D., Nicholson J. M. Mammalian tyrosinase: isolation by a simple new procedure and characterization of its steric requirements for cofactor activity. Arch Biochem Biophys. 1978 Jan 30;185(2):407–418. doi: 10.1016/0003-9861(78)90183-2. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jr, Ekel T. M., Montague P. M., Nicholson J. M. Mammalin tyrosinase. Stoichiometry and measurement of reaction products. Biochim Biophys Acta. 1980 Feb 14;611(2):251–268. doi: 10.1016/0005-2744(80)90061-3. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991 Nov;5(14):2902–2909. [PubMed] [Google Scholar]
- Janeway C. A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jekel P. A., Bak H. J., Soeter N. M., Vereijken J. M., Beintema J. J. Panulirus interruptus hemocyanin. The amino acid sequence of subunit b and anomalous behaviour of subunits a and b on polyacrylamide gel electrophoresis in the presence of SDS. Eur J Biochem. 1988 Dec 15;178(2):403–412. doi: 10.1111/j.1432-1033.1988.tb14464.x. [DOI] [PubMed] [Google Scholar]
- Johansson M. W., Keyser P., Söderhäll K. Purification and cDNA cloning of a four-domain Kazal proteinase inhibitor from crayfish blood cells. Eur J Biochem. 1994 Jul 15;223(2):389–394. doi: 10.1111/j.1432-1033.1994.tb19005.x. [DOI] [PubMed] [Google Scholar]
- Johansson M. W., Söderhäll K. Isolation and purification of a cell adhesion factor from crayfish blood cells. J Cell Biol. 1988 May;106(5):1795–1803. doi: 10.1083/jcb.106.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSON P., LIEBAU H. [On tyrosine metabolism of insects. V. Preparation in a pure state, crystallization and substrate specificity of o-diphenol oxidase from Calliphora erythrocephala]. Hoppe Seylers Z Physiol Chem. 1961 Nov 24;326:135–143. doi: 10.1515/bchm2.1961.326.1.135. [DOI] [PubMed] [Google Scholar]
- Kupper U., Niedermann D. M., Travaglini G., Lerch K. Isolation and characterization of the tyrosinase gene from Neurospora crassa. J Biol Chem. 1989 Oct 15;264(29):17250–17258. [PubMed] [Google Scholar]
- Kwon B. S., Wakulchik M., Haq A. K., Halaban R., Kestler D. Sequence analysis of mouse tyrosinase cDNA and the effect of melanotropin on its gene expression. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- Linzen B., Soeter N. M., Riggs A. F., Schneider H. J., Schartau W., Moore M. D., Yokota E., Behrens P. Q., Nakashima H., Takagi T. The structure of arthropod hemocyanins. Science. 1985 Aug 9;229(4713):519–524. doi: 10.1126/science.4023698. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Behrens P. Q., Moore M. D., Yokota E., Riggs A. F. Structure of hemocyanin II from the horseshoe crab, Limulus polyphemus. Sequences of the overlapping peptides, ordering the CNBr fragments, and the complete amino acid sequence. J Biol Chem. 1986 Aug 15;261(23):10526–10533. [PubMed] [Google Scholar]
- Nappi A. J., Vass E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993 Jun;6(3):117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Ochiai M., Ashida M. Purification of a beta-1,3-glucan recognition protein in the prophenoloxidase activating system from hemolymph of the silkworm, Bombyx mori. J Biol Chem. 1988 Aug 25;263(24):12056–12062. [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderhäll K., Aspán A., Duvic B. The proPO-system and associated proteins; role in cellular communication in arthropods. Res Immunol. 1990 Nov-Dec;141(9):896–907. doi: 10.1016/0923-2494(90)90190-a. [DOI] [PubMed] [Google Scholar]
- Söderhäll K., Cerenius L., Johansson M. W. The prophenoloxidase activating system and its role in invertebrate defence. Ann N Y Acad Sci. 1994 Apr 15;712:155–161. doi: 10.1111/j.1749-6632.1994.tb33570.x. [DOI] [PubMed] [Google Scholar]
- Söderhäll K. Prophenoloxidase activating system and melanization - a recognition mechanism of arthropods? A review. Dev Comp Immunol. 1982 Fall;6(4):601–611. [PubMed] [Google Scholar]
- Takase M., Miura I., Nakata A., Takeuchi T., Nishioka M. Cloning and sequencing of the cDNA encoding tyrosinase of the Japanese pond frog, Rana nigromaculata. Gene. 1992 Nov 16;121(2):359–363. doi: 10.1016/0378-1119(92)90144-e. [DOI] [PubMed] [Google Scholar]