Summary
Multi-dimensional mass spectrometry-based shotgun lipidomics (MDMS-SL) has become a foundation analytical technology platform among current lipidomics practices due to its high efficiency, sensitivity, and reproducibility, as well as its broad coverage. This platform has been broadly used to determine the altered content and/or composition of lipid classes, subclasses, and individual molecular species induced by diseases, genetic manipulations, drug treatments, and aging, among others. Herein, we briefly discussed the principles underlying this technology and presented a protocol for routine analysis of many of the lipid classes and subclasses covered by MDMS-SL directly from lipid extracts of biological samples. In particular, lipid sample preparation from a variety of biological materials, which is one of the key components of MDMS-SL was described in details. The protocol of mass spectrometric analysis can readily be expanded for analysis of other lipid classes not mentioned as long as appropriate sample preparation is conducted. It is our sincerely hope that this protocol could aid the researchers in the field to better understand and manage the technology for analysis of cellular lipidomes.
Keywords: Direct infusion, intrasource separation, lipidome, mass spectrometry, shotgun lipidomics
1. Introduction
Cellular lipids are a complex of special biological metabolites and can be classified into numerous categories, classes, and subclasses (1). They play distinct and critical roles in cellular functions. Lipids are the crucial components of cellular membranes, which constitute an impermeable barrier of cellular compartments and provide appropriate motifs for membrane protein function. Lipids serve as an energy storage depot. Many lipids serve as active second messengers. Lipidomics is a research field that studies cellular lipidomes on a large scale and at the intact molecular level (2, 3). Lipidomics research includes examination of the structures, functions, interactions, and dynamics of cellular lipidomes, and identification and quantification of thousands of individual lipid species.
Multi-dimensional mass spectrometry-based shotgun lipidomics (MDMS-SL) is a well-recognized technological platform to analyze individual lipid molecular species directly from lipid extracts of biological samples (4–6). The principle underlying the direct infusion-based MDMS-MS technology is to maximally exploit the unique chemical and physical properties of lipid classes in combination with the special advantages inherited in MS for lipid analysis, thereby achieving maximal separation and ionization, and minimal ion suppression. This principle is very different from that of the LC-MS approaches which maximally use the separation science of chromatography and is also different from other direct infusion-based shotgun lipidomics approaches which only employ some advantages of MS for lipid analysis. This principle and the comparison of its differences with other approaches have been extensively described in our review article (6). Here a summary of exploiting the unique properties of lipid classes and special advantages of MS is given.
Different lipid classes possess distinct hydrophobicities, reactivities, and stabilities. This unique feature allows us to separately prepare lipid samples targeted for the analysis of different categories or classes of lipids (we called it multiplexed extraction). For example, neutral lipids including triacylglycerol (TAG), non-esterified fatty acid (NEFA), cholesterol (Chol), and cholesterol ester (CE) can be readily extracted with hydrophobic solvents (e.g., hexane and ethyl ether) (7); acyl-CoA and gangliosides have to be extracted with relatively polar solvents (e.g., butanol) or with additional extraction repeats (8). It should be emphasized that incomplete extraction recovery of an individual lipid class can be compensated with the presence of an internal standard of the class and the effect of differential recoveries of individual lipid species of the class on quantification is only a secondary effect and can largely be ignored after a few repeated extractions (9). Sphingolipids are stable under alkaline conditions, which can be used to enrich and analyze this category of lipids (10). The instability of vinyl ether-containing lipids (i.e., plasmalogens) under acidic conditions is used to identify this category of lipids (11). The extracted lipids can be derivatized with a particular reagent to target a category of lipid classes containing an identical functional group in order to modify the polarity and/or to enhance the specific fragments of these lipid classes. For example, the primary amine in ethanolamine glycerophospholipid (PE) and lysoPE (LPE) can be readily tagged with fluorenylmethoxylcarbonyl (Fmoc) after addition of Fmoc chloride to the lipid solution. Then, the neutral loss of Fmoc from the Fmoc-tagged PE/LPE species in the negative-ion ESI-MS mode is very sensitive, showing a >15,000-fold linear dynamic range, which has been used to analyze PE and LPE species (12). It is well known that ionization of LPE species is severely suppressed by the co-existing abundant PE species after direct infusion of a lipid extract. However, with the Fmoc tagging, the increased linear dynamic range and the enhanced sensitivity for detection of the specific Fmoc fragment overcomes this ion suppression obstacle and makes the analysis of LPE species straightforward (12). Our recent success utilizing carnosine to tag very low abundance 4-hydroxyalkenal (4-HA) species is another example in the category (13).
Different lipid classes possess different charge properties, largely reflecting the nature of their polar head groups (2, 4). This unique feature allows us to use the electrospray ion source to selectively ionize a certain category of lipid classes under an experimental condition (we called it intrasource separation since this selective ionization is akin to electrophoretic separation) (4, 5, 14).
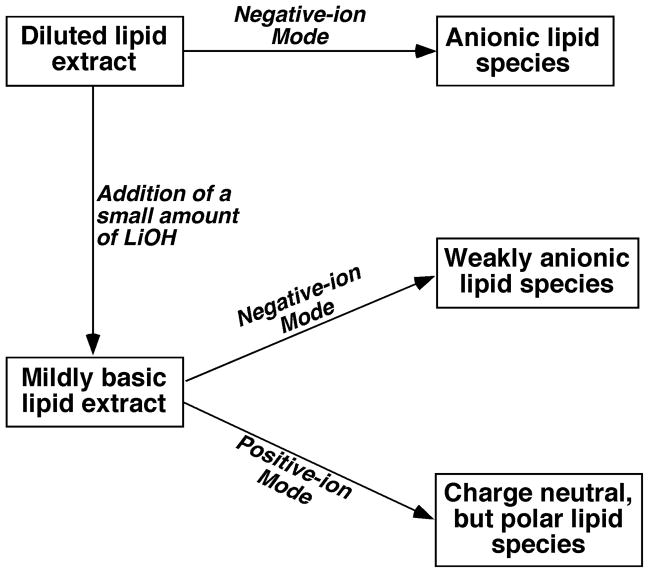
A strategy for intrasource separation of lipid classes from lipid extracts is illustrated (Figure 1) and has previously been discussed in detail (2, 4, 5, 14). After multiplexed extraction and intrasource separation, individual lipid molecular species of a class of interest can be ionized and displayed in an identical mass spectrum of a certain mass region regardless of the presence of ion suppression on the low abundance lipid classes from the abundant classes. This is a very important feature of shotgun lipidomics since all the species of a class are subject to be ionized under identical experimental conditions, which makes quantification of these species easier with the presence of internal standard(s) in comparison to liquid chromatography-mass spectrometry (LC-MS) where different experimental conditions are experienced for ionization of individual lipid species of a class.
Figure 1.
Schematic illustration of the experimental strategy for analysis of different lipid classes present in the biological samples.
After multiplexed extraction and intrasource separation, each ion peak in a mass spectrum may still represent 2 or more isomeric/isobaric lipid species, particularly those acquired with mass spectrometers having nominal mass resolution capabilities. Identification of these overlapped species is the task of multi-dimensional MS (i.e., MDMS) which analogs to MD-NMR, considering each variable as a dimension added to a full MS. Two useful variables (which can be combined as a building block variable in our case (see below)) for identification of individual lipid species are the fragment ions monitored by precursor ion scanning (PIS) and the neutral loss mass detected by neutral loss scanning (NLS). We recognized that the majority of biological lipid species are combinations of aliphatic chains, lipid backbones (e.g., glycerol, sphingosine, etc.), and/or head groups, each of which represents a building block of the lipid species under consideration. For example, 3 moieties linked to the hydroxyl groups of glycerol can be recognized as 3 building blocks. Identification of these building blocks will enable identification of each individual glycerol-derived lipid species in a given sample (4–6). An analogous approach can also be used to define other lipid classes (e.g., SM species in which the phosphocholine head group, the sphingoids (long chain bases), and the fatty acyl amides represent the 3 building blocks of each species) (6). Identification of these building blocks can be accomplished by the powerful MS/MS techniques (i.e., NLS and PIS). Therefore, all the building blocks of each lipid class constitute an additional dimension to the molecular ions present in the original mass spectrum, which is referred to as the first dimension. By correlating the peak of a given primary molecular ion in the first dimension with the building blocks in the second dimension, the structure(s) including regiospecificity as well as the isobaric constituents of the given molecular ion can be determined (5, 6). Again, the head group building block can be modified through derivatization in order to enhance its specificity and/or its ionization efficiency.
As the concentration of a lipid solution lower than the one to form lipid aggregation, ionization efficiencies of individual species of a class are essentially identical after de-isotoping of 13C isotopologues under identical experimental conditions (4, 15–17). This is a unique feature of shotgun lipidomics (see above). Based on this feature, we have developed a two-step quantification approach with selected internal standards in MDMS-SL after identification (4–6, 18). First, the abundant and non-overlapping species of a class are quantified by comparison with a pre-selected internal standard of the class after 13C de-isotoping. Next, some or all of the determined molecular species of the class (plus the pre-selected internal standard) are used as standards to determine the content of other low-abundance or overlapping molecular species using one or multiple MS/MS scans (each of which represents a specific building block of the class). Through this second step, the linear dynamic range of quantitation can dramatically be extended by eliminating background noise and by filtering the overlapping molecular species through a multi-dimensional approach (4, 19).
Based on these features, this platform can identify and quantify hundreds to thousands of individual lipid species of nearly 30 lipid classes (Table 1) in cellular lipidomes at its current stage of development, representing > 95% of the total lipid mass levels of a cellular lipidome directly from solvent extracts of biological materials from a limited amount of biological source materials (e.g., 10–50 mg of tissue, 106 cells, 200 μl body fluids, etc.) in an automated, unbiased, and relatively high-throughput manner (18, 19). Herein, we describe a primary protocol of the MDMS-SL platform for routine analysis of the majority of lipid classes without extensive derivatization. For analysis of many lipid classes for which derivatization is employed, the described protocol can be followed after sample preparation. The details can be found in original methodology papers. We believe that the MDMS-SL technology platform is powerful for comprehensive analysis of the majority of lipid classes present in cellular lipidomes and its utilization should bring to the development and/or improvement of diagnostics and therapeutics in humankind.
Table 1.
List of lipid classes covered by the MDMS-SL platform*
| Category | Class |
|---|---|
| Phospholipid | Cardiolipin (CL), phosphatidylcholine** (PC), phosphatidylethanolamine** (PE), dimethyl PE (DMPE), monomethyl PE (MMPE), phosphatidylserine (PS), phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylinositol (PI), lysoCL, lysoPC (LPC), lysoPE (LPE) |
| Sphingolipid | Hexosylceramide (HexCer), ceramide (Cer), sphingomyelin (SM), sulfatide (ST), sphingosine/sphinganine, sphingoid base-1-phosphate (S1P), psychosine, lysoSM |
| Glycolipid | Monohexosyl diacylglycereol (HexDAG) |
| Glycerolipid | Triacylglycerol (TAG) |
| Sterol | Cholesterol (Chol), cholesterol ester (CE), some oxysterols |
| Metabolites | Acylcarnitine, acyl-CoA, 4-hydroxyalkenal (4-HA), non-esterified fatty acid (NEFA) |
2. Materials
2.1 Equipment (see Note 1)
Nano-ESI source device (TriVersa NanoMate, Advion Bioscience Ltd., Ithaca, NY)
Mass spectrometer (Thermo TSQ VANTAGE, San Jose, CA)
2.2 Reagents
Chloroform (Burdick and Jackson, Muskegon, MI)
Methanol (Burdick and Jackson, Muskegon, MI)
Millipore deionized water
Lithium chloride (Sigma-Aldrich, St. Louis, MO)
Isopropanol (Burdick and Jackson, Muskegon, MI)
Lithium hydroxide (Sigma-Aldrich, St. Louis, MO)
BCA protein assay kit (Thermo Scientific, Rockford, IL)
1,2-Dimyristoleoyl-sn-glycero-3-phosphocholine (di14:1 PC) (All of the lipid internal standards are purchased from Avanti Polar Lipids, Inc., Alabaster, AL, except noted)
1,2-Dipalmitoleoyl-sn-glycero-3-phosphoethanolamine (di16:1 PE)
1,2-Dipentadecanoyl-sn-glycero-3-phosphoglycerol (sodium salt) (di15:0 PG)
1,2-Dimyristoyl-sn-glycero-3-phospho-L-serine (sodium salt) (di14:0 PS)
1,2-Dimyristoyl-sn-glycero-3-phosphate (sodium salt) (di14:0 PA)
1,1′,2,2′-Tetramyristoyl cardiolipin (T14:0 CL)
7,7,8,8-d4-Palmitic acid (d4-16:0 NEFA) (Cambridge Isotope Laboratories, Andover, MA)
N-Lauroryl sphingomyelin (N12:0 SM)
N-Heptadecanoyl ceramide (N17:0 Cer)
1-Heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (17:0 lysoPC)
1,2,3,4-13C4-Palmitoyl-L-carnitine hydrochloride (13C4-16:0 CN) (Sigma-Aldrich, St. Louis, MO)
Triheptadecenoin (T17:1 TAG) (Nu Chek, Inc. Elysian, MN)
3. Methods
3.1 Cellular lipid extraction
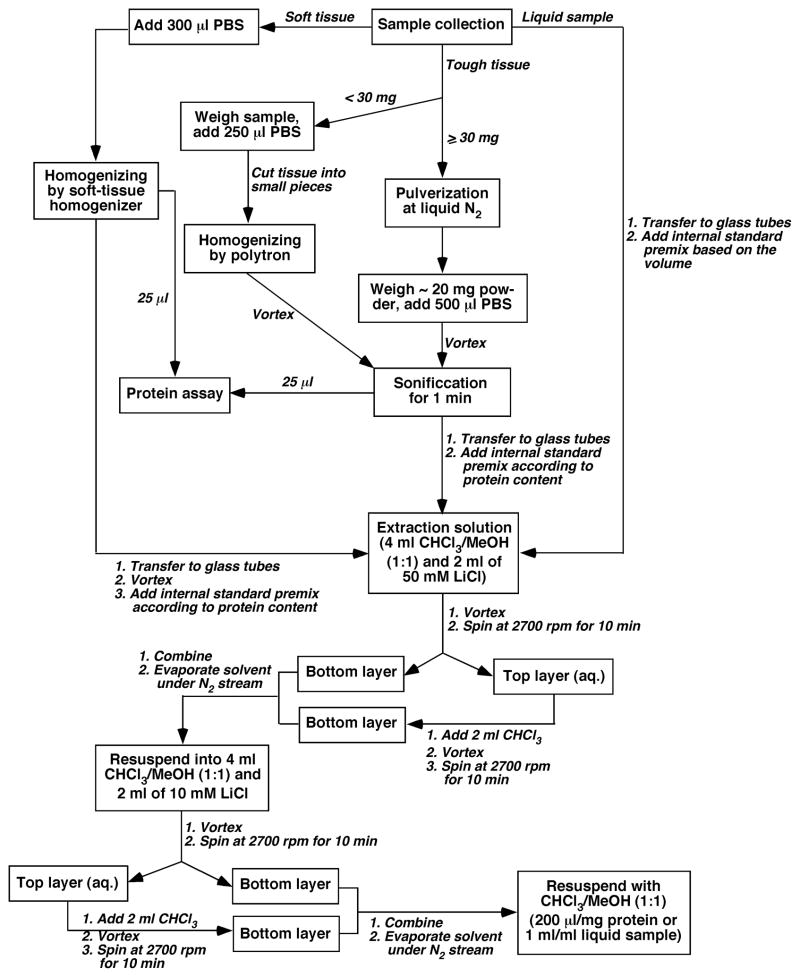
The workflow of this protocol is illustrated in Figure 2. Overall, tissue samples are homogenized in 10 times diluted phosphate buffered saline (PBS). The homogenates or other liquid samples are spiked with the appropriate internal standards in premixture. After a modified Bligh and Dyer extraction procedure, the resulting lipid extract is analyzed by ESI-MS and ESI-MS/MS.
Figure 2.
A schematic workflow of sample preparation for MDMS-SL analysis.
For the tissue samples which are not easily homogenized (e.g., heart, liver, kidney, and muscle samples). If the quantity of a sample is more than 30 mg, a whole piece of cryogenic frozen tissue is first pulverized by a stainless steel Bio-pulverizer under the temperature of liquid nitrogen. Weigh about 20 mg tissue fine powder in a cryogenic vial (2.0 mL), and add 500 μL of 10 times diluted PBS. Vortex the tubes before sonificantion of the samples for 1 min to disrupt tissue membranes (see Note 2). Pipet an aliquot of 25 μL for determination of protein content.
If the quantity of a sample is less than 30 mg, weigh the tissue samples in a cryogenic vial (2.0 mL), and add 750 μL of 10 times diluted PBS. Cut the tissue into small pieces using a piece of safety razor blade or a pair of scissors. Homogenize individual samples by a tissue-tearor utilizing an up-and-down dabbing motion for about 1 min, and then the homogenates are sonificated in a digital sonifier for 1 min to disrupt tissue cells (see Note 2). Pipet an aliquot of 25 μL for determination of protein content.
For soft tissue samples (e.g., cell pellet or brain samples). Keep the sample in a 1.5-mL Eppendorf tube. Add 300 μL of 10 times diluted PBS in the tube. The samples are homogenized for 1 min by using a disposable soft tissue homogenizer with an up-and-down dabbing motion (see Note 2). Pipet an aliquot of 25 μL for determination of protein content.
Protein assay is carried out by using a 96-well microplate and following the manufacturer’s instruction using bovine serum albumin as standard.
Accurately transfer individual homogenate of the tissue samples in step 1, 2 or 3 to a disposable culture borosilicate glass tube (16 × 100 mm) and record the transferred volume. Add a premixture of internal standards based on the protein content of the transferred homogenate (see Note 3).
For liquid samples (e.g., plasma, serum, or cerebrospinal fluid). The liquid is accurately transferred into a disposable culture borosilicate glass tube (16 × 100 mm) and record the transferred volume. Add a premixture of internal standards based on the volume of the transferred sample (see Notes 3 and 4).
Prepare extraction solvent, chloroform/methanol (1/1; v/v) (solvent A), and 10 and 50 mM lithium chloride solutions.
Add 4 mL solvent A to a glass tube for extraction, and an appropriate volume of 50 mM LiCl to bring the aqueous phase to a final volume of 2 mL. Cap the tubes and vortex them for 20 sec. The samples are then centrifuged at 2700 rpm for 10 min.
Collect the bottom layer to a new borosilicate glass tube (see Note 5). Add 2 mL chloroform to individual glass tubes with the residual top layer. Cap the tubes and vortex them for 20 sec. The samples are again centrifuged at 2700 rpm for 10 min.
Collect the bottom layer and combine it with that collected in Step 9 (see Note 5). Evaporate the combined bottom layer under a nitrogen stream with a nitrogen-evaporator until totally dried.
Resuspend individual residue in Step 10 with 4 mL of solvent A, and add 2 mL of 10 mM LiCl. Cap the tubes and vortex them for 20 sec. The samples are centrifuged at 2700 rpm for 10 min. Repeat Steps 9 and 10.
Resuspend individual lipid extract residue from Step 11 with solvent A in a volume of 200 μL/mg protein or 1 mL/mL original liquid samples. The lipid extracts are flushed with nitrogen, capped, and stored at −20 °C for MS analysis.
3.2 Mass spectrometric analysis of lipids
Lipid classes present in the prepared lipid samples are analyzed in three different modes as illustrated in Figure 1: negative-ion ESI, negative-ion ESI plus lithium hydroxide, and positive-ion ESI plus lithium hydroxide.
Dilute each lipid extract solution prepared to < 50 μM of total lipids with chloroform/methanol/isopropanol (1/2/4; v/v/v) with or without LiOH (2% to 5%) in a Teflon 96-well microplate (see Note 6).
Set the ionization voltage of the nanospray ionization source at 1.2 kV in the positive-ion mode, −1.2 kV in the negative-ion mode, and gas pressure at 2.0 psi. Nanospray ionization for each sample is performed by a customized sequence subroutine operated under the Chipsoft software (see Note 7).
For mass spectrometric analysis, collect 2-min duration of signal averaging in the profile mode for each survey MS scan (see Note 8). For tandem mass spectrometric analysis, set collision gas pressure at 1.0 mTorr, vary collision energy with the class of lipids, and collect a 5-min period of signal averaging in the profile mode for each tandem MS spectrum, including PIS and NLS, which are sensitive and specific to the lipid class or the category of lipid classes of interest, as show in Table 2. All of the mass spectra are automatically acquired by a customized sequence subroutine operated under Xcalibur software.
Table 2.
Summary of the specific scans in each lipid class used to identify and quantify individual molecular speciesa
| Lipid class (ref) | Ion format | Scans for class specific prescreen | Scans for identification of acyl chain and/or regioisomers | Preliminary scans for the second step quantitation |
|---|---|---|---|---|
| PC (25) | [M+Li]+ | NLS189.1, −35eV | NLS(59.0+FA), −40eV | NLS183.1, −35eV for polyunsaturated acyl chain containing species NLS59.0, −24eV for plasmalogen species NLS189.1, −35eV for all the other species |
| lysoPC (25) | [M+Na]+ | NLS59.0, −22eV NLS205.0, −34eV |
PIS104.1, −34eV PIS147.1, −34eV |
NLS59.0, −22eV NLS205.0, −34eV |
| PE, lysoPE (12) | [M−H]− [M−H+Fmoc]− ([M+C15H9O2]−) |
PIS196.1, 50eV for [M−H]− NLS222.2, 30eV |
PIS(FA-H), 30eV | NLS222.2, 30eV for [M−H+Fmoc]− |
| PI, lysoPI (14) | [M−H]− | PIS241.1, 45eV | PIS(FA-H), 47eV | PIS241.1, 45eV |
| PS, lysoPS (14) | [M−H]− | NLS87.1, 24eV | PIS(FA-H), 30eV | NLS87.1, 24eV |
| PG, PA, lysoPG, lysoPA (14) | [M−H]− | PIS153.1, 35eV | PIS(FA-H), 30eV | PIS153.1, 35eV |
| CL, mono-lysoCL (23) | [M−2H]2− | Full MS at high resolution | PIS(FA-H) at high resolution, 25eV; NLS(FA-H2O) at high resolution, 22eV | |
| TAG (21) | [M+Li]+ | NLS(FA), −35eV | ||
| Sphingomyelin (25) | [M+Li]+ | NLS213.2, −50eV | NLS(neutral fragments from sphingoid backbone) | NLS213.2, −50eV |
| Ceramide (26) | [M−H]− | NLS(neutral fragments from sphingoid backbone), (e.g., NLS256.2, 32eV for d18:1 non-hydroxyl species) | NLS(neutral fragments from sphingoid backbone), (e.g., NLS256.2, 32eV for d18:1 non-hydroxyl species) | NLS(neutral fragments from sphingoid backbone), (e.g., NLS256.2, 32eV for d18:1 non-hydroxyl species) |
| Hexosyl ceramide (27, 28) | [M+Li]+ | NLS162.2, −50eV | NLS(neutral fragments from sphingoid backbone) | NLS162.2, −50eV |
| Sulfatide (29) | [M−H]− | PIS 97.1, 65eV | NLS(neutral fragments from sphingoid backbone) | PIS97.1, 65eV |
| Sphingoid base-1-phosphate (30) | [M−H]− | PIS79.1, 24eV | PIS79.1, 24eV | |
| Sphingoid base (10) | [M+H]+ | NLS48.0, −18eV | NLS48.0, 18eV | |
| Psychosine (31) | [M+H]+ | NLS180.0, −24eV | NLS180.0, −24eV | |
| Cholesterol (32) | [cholesteryl methoxyacetate +MeOH+Li]+ | PIS97.1, −22eV | PIS97.1, −22eV | |
| Acyl carnitine (33) | [M+H]+ | PIS85.1, −30eV | PIS85.1, −30eV for all species; PIS145.1, −30eV for hydroxyl species | PIS85.1, −30eV |
| Acyl-CoA (34) | [M−H]−, [M−2H]2− [M−3H]3− |
PIS134.0, 30eV | PIS134.0, 30eV | PIS134.0, 30eV |
| 4-hydroxyalkenal (13) | [M+carnosine+H]+ | NLS71.2, −28eV NLS117.2, −26eV |
NLS71.2, −28eV NLS117.2, −26eV |
NLS and PIS stand for neutral-loss scan and precursor-ion scan, respectively. FA and (FA-H) denote free fatty acid and fatty acyl carboxylate anion, respectively. The abbreviations of phospholipid classes are given in the text.
3.3 Mass spectrometric data analysis
Data processing of MS analyses including ion peak selection, data transferring, baseline correction, peak intensity comparison, and quantification is conducted by self-programmed MicroSoft Excel macros (18). The principles of the macros are introduced as the following:
3.3.1 Establishment of the database of lipid classes and individual molecular species
The building block concept regarding lipid molecular structures is extensively employed to build the database of the program (18). On the basis of the differences of these building blocks, the majority of lipid classes present in the mammalian cellular lipidomes are classified into five categories, including glycerophospholipids, glycerolipids, sphingolipids, sterols, and metabolites (see Note 9).
All lipid classes are defined by backbones and building blocks. For example, choline glycerophospholipids (PC) is one class of lipids in the glycerophospholipid category. It has glycerol as its backbone with three building blocks connected the three hydroxy groups. The phosphodiester choline head group at the sn-3 position specifies the class. The oxygen atom of glycerol at the sn-1 position is connected to an aliphatic chain through an ester, ether, or vinyl ether bond, which defines the phosphatidyl-, plasmanyl-, and plasmenyl-PC, according to the IUPAC nomenclature (see Note 10). The oxygen atom at the sn-2 position is connected to the other aliphatic chain through an ester bond.
The building blocks of fatty acyl chain vary with the number of carbon atoms and the number of double bonds, as well as the location of the double bonds in the aliphatic chains. The variations of the carbon atom number and double bond number in the aliphatic chains composite of the entire lipid class, such as PC, in the database of lipid classes for MDMS-SL analysis (see Note 11).
3.3.2 Automated data processing to identify and quantify individual molecular species of a class of interest present in a cellular lipidome
Tabular raw data from the mass spectra are transferred by self-programmed software directly from the Xcalibur platform.
The baseline levels of the tabular raw data from the mass spectra are the sum of baseline drift and noise level. The baseline drift is determined by averaging a number of lowest ion intensities. The noise level is determined based on the fact that an accelerated intensity change exists from noise to signal (20). The precisely determined baseline level is deduced from the raw data for identification and quantification of analytes.
An ion peak list of the molecular species in a lipid class of interest present in the analyzed lipid extract is generated by matching the m/z values of the detected ion peaks after baseline correction in the specific scan (i.e., PIS or NLS, Table 2) with those of the candidate species in the established database of the lipid class of interest. This peak list represents all the detectable species of the specific class including isomeric species, and contains information about the total number of carbon atoms and the total number of double bonds of the aliphatic chain(s) from the lipid database of the program.
Identification of acyl chain moieties is achieved by loading all PIS or NLS data specific for acyl chain information. The combination of the paired aliphatic chains is determined by the restriction of the total number of carbon atoms and the total number of double bonds present in the acyl chains identified for each individual species.
Before direct quantification of the class of lipid molecular species of interest, the effects of 13C isotope need to be considered (4, 21). There exist two types of these effects. The first type of effect comes from the carbon number difference between a given molecular species and the selected internal standard. The second type of effect is because of the overlapping of the ion peak of the species of interest (m/z = M) with the 13C isotope peak of another species containing an additional double bond (m/z = M−2) (See Note 12).
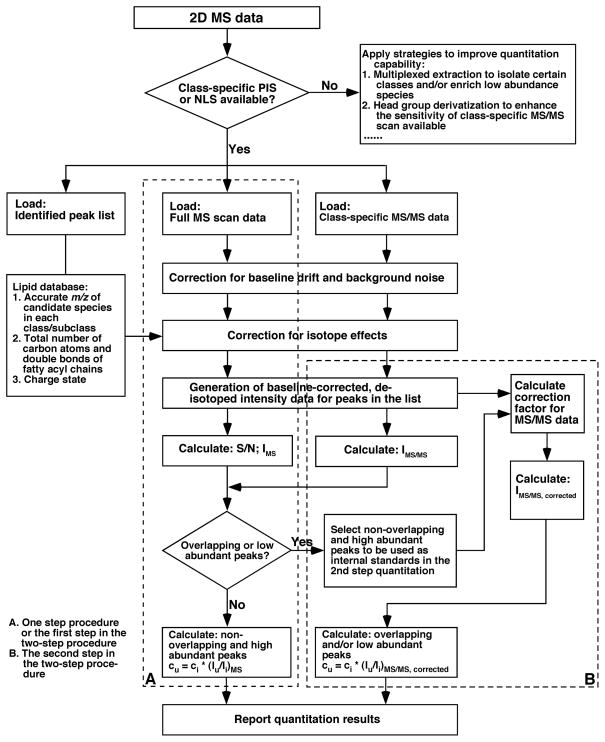
Quantification of the identified individual molecular species is performed in a two-step procedure, as shown by the workflow in Figure 3. An algorithm first determines whether there exist overlapping or low-abundance peaks in the peak list of interest. The step one quantification is performed for the abundant and non-overlapping peaks by direct ratiometric comparison to the ion peak intensity of the selected internal standard of the class in the survey MS scan after baseline correction and removal of 13C isotope effects (route A in Figure 3).
The determined non-overlapping and abundant species plus the exogenously added internal standard are the candidate standards for the second step of quantification. The corrected ion peak intensities of the overlapping and/or low-abundance species from the class-specific PIS or NLS (Table 2) are used to quantify these species with ratiometric comparison with the ion-peak intensities of the candidate standards (route B in Figure 3). (See Note 13)
Figure 3.
A schematic workflow used in MDMS-SL to quantify individual molecular species in a lipid class of interest (Reproduced with permission from reference (18)).
Acknowledgments
This work was supported by National Institute on Aging Grants R01 AG31675 and intramural institutional research funds. Special thanks are expressed to Ms. Imee Tiu for her editorial assistance.
Footnotes
The nanospray source is controlled by Chipsoft 8.3.1 software. All MS or tandem MS analyses are operated under the Xcalibur software. Additional equipments and supplies needed include analytical balance (readability 0.01 mg), multi-sample bio-pulverizer (12 wells, capacity 10–100mg per well), cryogenic vials (2.0 mL), Branson digital sonifier 450, vortex shaker and mixer, razor blade or scissors, tissue tearor, 1.5-mL Eppendorf tubes, 1.5 mL polypropylene pestles (disposable soft tissue homogenizer) with handheld pellet pestle motor, disposable culture borosilicate glass tubes (16 × 100 mm), 5.75″ disposable borosilicate glass Pasteur pipets, Drummond pipet-aids, table top centrifuge, analytical nitrogen evaporator, 96-well microplates (transparent for protein assay and chemical resistance for preparing lipid samples for direct infusion).
During sonification or homogenization, samples are kept in an ice bath to keep cold.
The internal standards mixture includes di14:1 PC, di16:1 PE, di15:0 PG, di14:0 PS, di14:0 PA, T14:0 CL, d4-16:0 NEFA, N12:0 SM, N17:0 Cer, 13C4-16:0 CN, 17:0 lysoPC, T17:1 TAG, etc. The stock solution of individual internal standard is prepared in either solvent A or pure chloroform with a concentration approximately 1 mg/mL. The amount of each individual lipid species in the premixture is prepared based on the abundance of the corresponding lipid class in the samples. The molecular species of internal standards are selected because they represent < 0.1% of the endogenous cellular lipid mass levels as predetermined by ESI-MS lipid analysis.
Alternatively, liquid samples can also be normalized to their protein contents. In this case, an aliquot of the liquid sample is used to determine the protein content prior to addition of the internal standard premixture.
In order to eliminate the contamination from the top layer (aqueous phase) to the bottom phase, insert the glass Pasteur pipet into the upper layer by slowly air bubbling until the pipet inserts into the bottom layer, which could avoid the upper layer liquid going into the pipet. When taking the glass pipet out from the upper layer, touch the outside of the pipet on the edge of the glass tube and quickly transfer the bottom layer to a clean glass tube.
The total lipid concentration of a lipid extract can be estimated on the basis of the protein content or previous experience (22). This knowledge is useful for estimation of the concentrations of total lipids to prevent lipid aggregation during analysis. The lithium hydroxide is made of 200-time dilution of a saturated methanol solution.
Since sample ionization and spectra collection are operated with two separate software programs (i.e., ChipSoft and Xcalibur, respectively), the ionization polarity and time controlled by the ChipSoft should be matched to those of the mass spectrometer. The mass spectrometer will be triggered to start collecting spectra with the start of the nanospray.
For the triple-quadrupole mass spectrometer, the first and third quadrupoles are used as independent mass analyzer with a mass resolution of 0.7 Th, and the second quadrupole serves as a collision cell for tandem mass spectrometry. For the analysis of cardiolipin, the mass resolution is set at 0.3 Th to detect its doubly-charged ions (23).
MDMS-SL allows us to analyze lipid molecular species of a class of interest in a non-targeted approach. Therefore, the database should be as broad and flexible as possible. The former covers all possible natural lipid molecular species, whereas the latter allows to modify or expand the database as necessary.
To date, the plasmanyl and plasmenyl subclasses have been identified only in choline, ethanolamine, and serine glycerophospholipids in mammalian lipidomes (24).
The molecular species that are included in our database are approximately 6,500 glycerophospholipid species, 3,200 glycerolipid species, 26,000 sphingolipid species, 100 sterol lipids, and 410 metabolites (18). Therefore, a total of over 36,000 molecular species, not counting regioisomers, oxidized lipids, or other covalently modified entities, are included in the initial construction of the database. Moreover, by modifying the general chemical formulas, the constructed databases can easily be extended to cover any new species or subclasses in each lipid class when the sensitivity of mass spectrometers is further improved or any unusual lipid profiles are analyzed from a biological sample.
The isotope effects from other atoms, such as hydrogen, nitrogen, or phosphorus, are usually neglected due to extremely low abundance of its isotope or no difference between the species and the selected internal standard.
An algorithm could be generated based on two variables (i.e., the differences in the number of total carbon atoms and the number of total double bonds present in fatty acyl chains of each individual species from those of the selected standards) with multivariate least-square regression to determine the correction factors for each individual molecular species for the second-step quantification (18). With this second step, the linear dynamic range of quantification is extended dramatically to quantify overlapping and/or low-abundance species with one or more MS/MS scans to reduce background noise, increase S/N ratios of low-abundance species, and filter the overlapping molecules with class-specific PIS or NLS.
References
- 1.Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Shevchenko A, Simons K. Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol. 2010;11:593–598. doi: 10.1038/nrm2934. [DOI] [PubMed] [Google Scholar]
- 4.Han X, Gross RW. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of the cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 5.Han X, Gross RW. Shotgun lipidomics: multi-dimensional mass spectrometric analysis of cellular lipidomes. Expert Rev Proteomics. 2005;2:253–264. doi: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- 6.Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of cellular glycerophospholipids enabled by multiplexed solvent dependent analyte-matrix interactions. Anal Chem. 2008;80:7576–7585. doi: 10.1021/ac801200w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiebish MA, Bell R, Yang K, Phan T, Zhao Z, Ames W, Seyfried TN, Gross RW, Chuang JH, Han X. Dynamic simulation of cardiolipin remodeling: greasing the wheels for an interpretative approach to lipidomics. J Lipid Res. 2010;51:2153–2170. doi: 10.1194/jlr.M004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang K, Han X. Accurate Quantification of Lipid Species by Electrospray Ionization Mass Spectrometry — Meets a Key Challenge in Lipidomics. Metabolites. 2011;1:21–40. doi: 10.3390/metabo1010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Cheng H, Yang K, Gross RW, Han X. Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low abundance regime of cellular sphingolipids. Anal Biochem. 2007;371:135–145. doi: 10.1016/j.ab.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang K, Zhao Z, Gross RW, Han X. Shotgun lipidomics identifies a paired rule for the presence of isomeric ether phospholipid molecular species. PLoS ONE. 2007;2:e1368. doi: 10.1371/journal.pone.0001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Yang K, Cheng H, Fikes KN, Gross RW. Shotgun lipidomics of phosphoethanolamine-containing lipids in biological samples after one-step in situ derivatization. J Lipid Res. 2005;46:1548–1560. doi: 10.1194/jlr.D500007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Fang H, Han X. Shotgun lipidomics analysis of 4-hydroxyalkenal species directly from lipid extracts after one-step in situ derivatization. Anal Chem. 2012;84:4580–4586. doi: 10.1021/ac300695p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Yang J, Cheng H, Ye H, Gross RW. Towards fingerprinting cellular lipidomes directly from biological samples by two-dimensional electrospray ionization mass spectrometry. Anal Biochem. 2004;330:317–331. doi: 10.1016/j.ab.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc Natl Acad Sci USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koivusalo M, Haimi P, Heikinheimo L, Kostiainen R, Somerharju P. Quantitative determination of phospholipid compositions by ESI-MS: effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J Lipid Res. 2001;42:663–672. [PubMed] [Google Scholar]
- 17.DeLong CJ, Baker PRS, Samuel M, Cui Z, Thomas MJ. Molecular species composition of rat liver phospholipids by ESI-MS/MS: the effect of chromatography. J Lipid Res. 2001;42:1959–1968. [PubMed] [Google Scholar]
- 18.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multi-dimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Yang K, Gross RW. Microfluidics-based electrospray ionization enhances intrasource separation of lipid classes and extends identification of individual molecular species through multi-dimensional mass spectrometry: Development of an automated high throughput platform for shotgun lipidomics. Rapid Commun Mass Spectrom. 2008;22:2115–2124. doi: 10.1002/rcm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Fang X, Gross RW, Han X. A practical approach for determination of mass spectral baselines. J Am Soc Mass Spectrom. 2011;22:2090–2099. doi: 10.1007/s13361-011-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X, Gross RW. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2001;295:88–100. doi: 10.1006/abio.2001.5178. [DOI] [PubMed] [Google Scholar]
- 22.Christie WW, Han X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis. 4. The Oily Press; Bridgwater, England: 2010. [Google Scholar]
- 23.Han X, Yang K, Yang J, Cheng H, Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J Lipid Res. 2006;47:864–879. doi: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance DE, Vance JE. Biochemistry of Lipids, Lipoproteins and Membranes. 5. Elsevier Science B.V; Amsterdam: 2008. [Google Scholar]
- 25.Yang K, Zhao Z, Gross RW, Han X. Systematic analysis of choline-containing phospholipids using multi-dimensional mass spectrometry-based shotgun lipidomics. J Chromatogr B. 2009;877:2924–2936. doi: 10.1016/j.jchromb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X. Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;302:199–212. doi: 10.1006/abio.2001.5536. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Cheng H. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J Lipid Res. 2005;46:163–175. doi: 10.1194/jlr.D400022-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Hsu FF, Turk J. Structural determination of glycosphingolipids as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisional-activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom. 2001;12:61–79. doi: 10.1016/S1044-0305(00)00194-X. [DOI] [PubMed] [Google Scholar]
- 29.Hsu FF, Bohrer A, Turk J. Electrospray ionization tandem mass spectrometric analysis of sulfatide. Determination of fragmentation patterns and characterization of molecular species expressed in brain and in pancreatic islets. Biochim Biophys Acta. 1998;1392:202–216. doi: 10.1016/s0005-2760(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Han X. Characterization and direct quantitation of sphingoid base-1-phosphates from lipid extracts: A shotgun lipidomics approach. J Lipid Res. 2006;47:1865–1873. doi: 10.1194/jlr.D600012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Yang K, Han X. Direct quantitation of psychosine from alkaline-treated lipid extracts with a semi-synthetic internal standard. J Lipid Res. 2009;50:162–172. doi: 10.1194/jlr.D800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng H, Jiang X, Han X. Alterations in lipid homeostasis of mouse dorsal root ganglia induced by apolipoprotein E deficiency: A shotgun lipidomics study. J Neurochem. 2007;101:57–76. doi: 10.1111/j.1471-4159.2006.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005;44:5234–5245. doi: 10.1021/bi047773a. [DOI] [PubMed] [Google Scholar]
- 34.Kalderon B, Sheena V, Shachrur S, Hertz R, Bar-Tana J. Modulation by nutrients and drugs of liver acyl-CoAs analyzed by mass spectrometry. J Lipid Res. 2002;43:1125–1132. doi: 10.1194/jlr.m200060-jlr200. [DOI] [PubMed] [Google Scholar]