Abstract
Xenoestrogen mimics estrogen-like activities primarily based on alterations of gene expression and interactions with estrogen receptors ERα and ERβ. However, requirement for large concentrations to induce estrogenic phenotypes and low affinity for ERs have challenged the notion that prevailing xenoestrogens are significant health hazards. Here, in this study we show that under certain conditions, exposure of xenoestrogen could be potentially harmful in respect of enhanced uterine estrogenicity. Previously, we have demonstrated that estradiol-17β (E2) upregulates uterine Bip, a stress-related endoplasmic reticulum protein, via ER-independent mechanism in mice. Moreover, this protein essentially involves in E2-mediated uterine growth response and ERα-dependent gene transcription. Here, we demonstrate that among three tested xenoestrogens, only kepone (>15-30 mg/kg) exerts sustained inductive response for uterine Bip expression. Interestingly, this kepone-induced Bip strongly correlates with ERα-dependent growth and gene expressional responses in the mouse uterus. Furthermore, these effects were strongly suppressed after knock-down of uterine Bip, via adenovirus approach. While, kepone at 7.5 mg/kg was not effective, but was strongly stimulatory by adenovirus-driven forced expression of uterine Bip. In contrast, the control GFP virus was not effective in above responses. Furthermore, the induction of uterine Bip by stress-related signals also revealed the onset of uterine growth in mice, when exposed to sub-lethal dose of kepone. Collectively, studies provide novel molecular evidence that Bip acts as a critical regulator to amplify estrogenic potency for a weak xenoestrogen kepone.
Although banned in the industrial nations, organochlorine compounds, such as pesticides and polychlorinated biphenyls are still used in the third world countries (1-3), and are highly persistent organic pollutants that have been identified in diverse environmental conditions worldwide (4-8). This has been a major issue about the potential health and environmental risks associated with the exposure to human and wildlife (9, 10), by disrupting endocrine system and affecting normal functions of hormone-responsive target tissues. In particular, the harmful effects of many organochlorine compounds in reproductive organs have been suspect for many years, but the mode and extent of their actions are not clearly defined (11-13).
In general, they have been attributed to their ability to mimic certain effects of primary estrogens, thus cataloging them as xenoestrogens (14, 15). In particular, they have drawn attention because of their ability to interact with nuclear estrogen receptor-α and -β (ERα and ERβ). It has been argued, nonetheless that the extent of their effects is minimal because of low binding affinities for ERs (16-18), and the need for larger molar concentrations in order to produce a phenotypic effect (19-22). However, there is also evidence that response to an “estrogen” in a target tissue is not necessarily related to its affinity for the receptor (23, 24). For example, doisynolic acid shows uterotrophic activity in the rat similar to that induced by estradiol-17β (E2), yet shows little affinity for ERs (25, 26). Moreover, estrogenicity of xenobiotics may involve various steroid-binding protein pathways; they may interact with ER or other binding proteins that may not result in the similar kind of transactivation that normally occurs with natural ligands. The metabolic conversion of xenobiotics may also alter their estrogenic activity. For example, the pesticide methoxychlor is a more potent reproductive toxicant when given orally than when injected (27, 28). Which of the many metabolites that are important has yet to be established, but a recent comparison of the affinity of some metabolites for the ER indicates a span of four orders of magnitude (16). Furthermore, xenobiotics can be effective at very low doses comparable to their levels of exposure in humans and wildlife (29, 30). Additionally, xenoestrogens exhibit significant differences in respect of coactivator recruitment and transcriptional activation, as compared to the natural estrogens (31-34).
Given their physicochemical differences and distinct biological effects, it is not surprising that a variety of mechanisms are used by endocrine disrupting chemicals. Understanding molecular mechanisms of estrogen and xenoestrogen action will, therefore, be of considerable utility in many areas. Because uterine sensitivity to estrogenic substances is reflected in well-characterized biological responses that culminate to increased uterine tissue weight and epithelial cell proliferation, xenoestrogen-mediated uterotrophic assays have been used to assess the potency of estrogenic actions. While xenoestrogen may utilize both ERα and ERβ, in the mouse uterus ERα has been shown to be a major receptor for the control of estrogen-dependent growth response (35-37). However, little is known regarding mechanism of actions of xenoestrogen at the molecular level, although accumulated evidence suggest that xenoestrogens induce estrogenic phenotypes with or without involving ER (20-22, 38).
We have previously shown that Bip (also known as grp78 encoded by Hspa5), a member of heat shock protein 70 (HSP70) family, is induced early by natural estrogens in the mouse uterus via ER-independent mechanism (39). Furthermore, this protein plays an essential role in the regulation of estrogen-dependent ERα-mediated gene transcription and growth responses in the uteri of mice (40). Bip is a resident protein in the endoplasmic reticulum (ER) where it binds with newly synthesized peptide chains during the processes of secretion and translocation of proteins (41, 42). Here, we examined the regulation and functional activities of Bip in xenoestrogen-mediated effects in the uteri of mice. Studies provide evidence that kepone (> 15 mg/kg), among three xenoestrogens, specifically induces sustainable levels of uterine Bip without involving ER, but facilitates complex formation between Bip and ERα in order to control ERα-dependent uterine gene expression. These results are consistent with the increase in uterine growth by kepone in the wild-type, but not in ERα null mice. Remarkably, heightened accumulation of uterine Bip, either by adenovirus-driven strategy or stress response, causes simulation of uterine cell proliferation by sub-optimal dose of kepone in mice, suggesting Bip acts as a critical regulator to determine estrogenicity for a weak xenoestrogen kepone.
Materials and Methods
Animals and tissue preparation
Adult CD-1 (Charles River Laboratories, Wilmington, MA) mice were housed in our institutional animal care facility according to NIH and institutional guidelines for laboratory animals. In general, 8 to 10 wk old adult mice were ovariectomized and rested for 7 days before they received any injections. They received a single subcutaneous (sc) injection (0.1 ml/mouse) with sesame seed oil (control), E2 (4 μg/kg) (positive control), kepone (7.5, 15 or 30 mg/kg), methoxychlor (7.5, 15 or 30 mg/kg) or o,p’-DDT (7.5, 15 or 30 mg/kg) and killed at indicated time points. In addition, one group of mice was subjected to daily single injection of kepone (7.5 mg/kg) for 3 consecutive days, and killed 24 h after the last injection. All the compounds were dissolved in absolute ethanol and diluted to the desired concentrations in sesame seed oil. Tissues were rapidly flash-frozen and kept at −70°C, or fixed in 10% formaldehyde before paraffin embedding for subsequent studies.
In some studies, wild-type and ERα(−/−) littermates (43) were analyzed in parallel. Both mice (C57BL/6J/129/J) were produced by crossing of heterozygous females and males in our animal facility. These mice were also given a single injection (sc) of oil, kepone (15 or 30 mg/kg), ICI (20 mg/kg) or the same dose of ICI 30 min prior to kepone. Mice were killed 24h after the last injection. Tissues were collected as above.
In another set of experiments, ovariectomized wild-type mice were given injections of kepone (2.5 and 7.5 mg/kg) or oil (as vehicle control), and immediately subjected to physiologic stress using an ultrasonic sound repellant device (Victor Sonic PestChaser, Woodstream, PA) or direct application of endoplasmic reticulum stress inducer, tunicamycin (1.0 mg/kg, sc). Mice were killed after 24 h and uterine tissues were collected for analysis.
Probes and Northern blot hybridization
Complementary-RNA (c-RNA) probes were generated from mouse-specific cDNA clones. The cDNA clones of Bip, SFRP-2 (secreted frizzled related protein 2), Wnt4, Wnt5a and rpL7 (ribosomal protein L7) have previously been described (39, 44). For northern blot hybridization, 32P-labeled antisense c-RNA probes were generated using appropriate RNA polymerases. The probes had specific activities of 2×109 dpm/μg. Northern blot hybridization technique was same as previously described (45). Stripping of hybridized probe before subsequent rehybridization was achieved as described (45) Transcripts were detected by autoradiography. The abundance of mRNAs for each gene expression was quantitated by analysis of band intensities on the autoradiogram using densitometric scanning and was corrected against rpL7.
Antibodies and Other Reagents
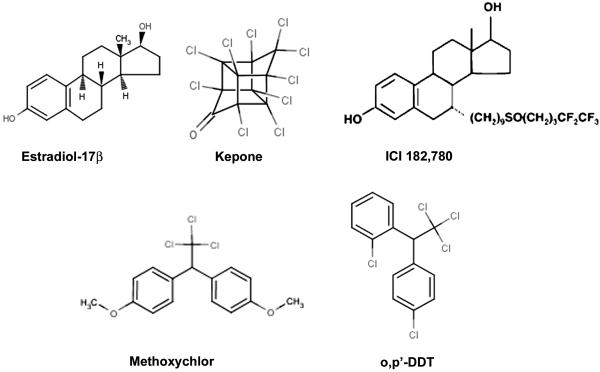
The affinity-purified polyclonal antibodies for Bip (catalog no. sc-1050), ERα (cat# sc-542), actin (cat# sc-1615), PR (cat# sc-539), and GFP (cat# sc-1050) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Ki-67 antibody (cat# NCL-Ki67 paraffin) was purchased from Novocastra Laboratories Ltd., (Newcastle upon Tyne, UK). Bromodeoxyuridine (BrdU) (cat# B9285), estradiol-17β (E2) and methoxychlor (DMDT; 1,1,1,-Trichloro-2,2,-bis-[p-methoxyphenyl]ethane) were purchased from Sigma Chemical Co. (St. Louis, MO). Kepone (chlordecone) [1,1a,3,3a,4,5,5a,5b,c-decachlorooctoahydro-1,3,4-metheno-2H-cyclobuta (cd) pentalen-2-one] and o,p’-DDT [1-(o-chlorophenyl)-1-(p-chlorophenyl) 2,2,2-trichloroethane] were purchased from Cerilliant Corporation, (Austin, TX) and were at least 99% pure. ICI-182,780 (ICI) [7 (9-4,4,5,5,5-pentafluoropenylsulfinyl) nonyl-estra-1,3,4(10-triene-3,17 -diol)] was a gift of Zeneca Phamaceuticals (Cheshire, England). Chemical structures of kepone, o,p’-DDT, methoxychlor, E2 and ICI are shown in Fig. 1.
FIG. 1.
Chemical structures of the natural estrogen estradiol-17β (E2), antiestrogen ICI 182,780 and the oganochlorine pesticides kepone, methoxychlor and o,p-DDT used in the present study.
Immunohistochemistry, Immunoprecipitation, and Western Blotting
These procedures were same as previously described (46). For studies with immunoprecipitation and Western blotting, proteins were extracted in Tissue-PE LB lyses buffer (Geno Tech). For studies with BrdU incorporation into DNA, mice were injected with BrdU (50 mg/kg, ip), 2h before they killed. Formaldehyde fixed paraffin-embedded tissue sections were stained for BrdU incorporation, using biotinylated antibody according to the manufacturer’s instruction (catalog no. 93-3944; Zymed laboratories Inc., San Francisco, CA).
Recombinant adenoviral plasmids and generation of viral particles
The generation of recombinant adenoviral plasmids for the antisense Bip (rAdBipAs) and GFP control (rAdGFP) was previously described (40). The recombinant Bip-sense cDNA construct (rAdBipS) was generated as follows. The full-length coding region of mouse Bip cDNA was generated by RT-PCR using primers carrying linkers for EcoRV at 5′-ends of both sense: 5′-GCCCCGGGATGATGAAGTTCACTGTG-3′ and antisense: 5′-GCCCCGGGCTACAACTCATCTTTTTC-3′. The amplified DNA fragment was inserted into a shuttle vector pAd-track CMV at EcoRV site, in a direction of sense with respect to CMV promoter. The identity of the clone was confirmed by sequencing. This clone DNA was linearized with PmeI and subsequently cotransfected with pAdEasy-1 into E. coli BJ5183 to obtain the recombinant clone. The recombinant plasmid clones harboring either Bip-S or Bip-As DNA possess an additional CMV promoter which drives green fluorescence protein (GFP) independently. The viral packaging of these plasmids was carried out by transfection into 293 cells as described (47). Viral particles were purified through CsCl density gradient centrifugation and stored at −70°C.
In vivo virus delivery
This was followed essentially same as previously described (40). In brief, adenoviral particles were first inoculated directly into uterine lumen of both horns (20 μl solutions in saline containing 1×1011 virus particles per horn) from the oviduct-end just before ovariectomy. They were given rest for 7 days before they received the second inoculum (~ 100 μl solution in saline containing 1 × 1011 virus particles) through tail vein. They were again rested for 2 more days prior receiving injections of kepone or oil (as vehicle control) for 24 h. Uterine tissues were appropriately collected for subsequent analysis.
RT-PCR
Procedures for the RT and comparative PCR followed the previously described methods (48, 49) with some modifications. In brief, total RNA was extracted from mouse uterus using Trizol according to the manufacturer’s instruction. RT with oligo-dT priming was performed to generate cDNAs from 4 μg total RNA using Superscript II following the instruction provided by the manufacturer. DNA amplification was carried out with Taq DNA polymerase (Invitrogen, San Diego, CA) using the following primers: cyclin D1 (329 bp), 5′-GCGTACCCTGACACCAATCT-3′ and 5′-CACAACTTCTCGGCAGTCAA-3′; Mad2 (182 bp), 5′-TCCCTACAGACACCCTCCAC-3′ and 5′-TTCTTGCGCTTCTGGAAGAT-3′; rpL7 (246 bp), 5′-TCAATGGAGTAAGCCCAAAG-3′ and 5′CAAGAGACCGAGCAATCAAG-3′. PCR conditions were 94°C for 4 min and then appropriate number of cycles (as indicated in figures for genes of interest) for linear amplification using 94°C for 30 sec, 55°C for 30 sec, and 72°C for 45 sec, followed by incubation at 72°C for 10 min. Amplified fragments were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. The intensity of each band was measured by Scion Image (Scion Corp., Frederick, MD), and the abundance of mRNAs for each gene expression was corrected against rPL7.
Results
Kepone selectively modulates Bip and maintains its sustained upregulation in a dose dependent manner in the mouse uterus
Previously, we demonstrated that Bip and Wnt-signaling genes are regulated by E2 in the mouse uterus (39, 44). Here, we examined whether xenoestrogens viz. kepone, methoxychlor and o,p’-DDT also possess similar responses to these uterine genes. Based on previous reports with respect to the doses used for these compounds for studies with regulation of uterine genes (13, 20-22), we tested all three xenoestrogenic compounds in mice using doses 7.5, 15.0 and 30.0 mg/kg for 24h. Northern blot hybridization analyses revealed that kepone (Fig. 2A), similar to E2 (Fig. 2D), was capable of inducing uterine Bip mRNA expression (3-4 fold) at or above 15 mg/kg over the control (oil). In contrast, o,p’-DDT (Fig. 2B) or methoxychlor (Fig. 2C) was unable to show such response. In addition, none of these xenoestrogenic compounds (Fig. 2A-C) had any effects on SFRP-2, a Wnt-signaling antagonist. While consistent to previous results (39), this gene was suppressed by E2 (Fig. 2D). Similarly, other E2-responsive Wnt signaling genes (viz., Wnt4, Wnt5a and β-catenin) (44), were also not affected by these xenoestrogenic compounds at above doses (data not shown). Overall, these results suggest that uterine Bip is specifically altered by kepone in an estrogen-responsive manner and provides a basis for further investigation of kepone-dependent regulations.
Fig. 2. Uterine regulation of gene specific mRNAs by xenoestrogenic compounds and E2. Dose response studies. Adult ovariectomized wild-type mice were injected (sc) with (A), kepone (7.5, 15 or 30 mg/kg); (B), o,p’-DDT (7.5, 15 or 30 mg/kg); (C) methoxychlor (7.5, 15 or 30 mg/kg) or (D), E2 (4 μg/mouse) (as a positive control) for 24 h. Mice injected with oil and killed after 24 h was served as vehicle control.
Total RNA (6 μg) was separated by formaldehyde-agarose gel electrophoresis, transferred and UV cross-linked to nylon membrane, and hybridized as described in the Materials and Methods. Hybridization was performed using 32P-labeled cRNA probes sequentially to Bip, SFRP-2 (secreted frizzled related protein 2) and rpL7 (ribosomal protein L7). Representative autoradiograms are shown with exposures at 5 h for Bip, 15 h for SFRP-2 and 3 h for rpL7. Temporal studies. Uterine total RNA (6 μg in each lane) was analyzed at indicated times, using kepone at 7.5 mg/kg (E) or 30 mg/kg (F) or E2 (4 μg/mouse) (G). Hybridization was performed using 32P-labeled cRNA probes sequentially to Bip, and rpL7. Autoradiographic exposure times were similar as indicated above. In general, 3-5 mice were used for each group analysis. These experiments (A-G) were repeated three times with independent RNA samples. In the bar plot, the abundance of mRNAs for each gene expression was quantitated by analysis of band intensities using densitometric scanning and was corrected against rpL7. *Values are statistically different (p<0.05, Student’s t test) from the oil treated group.
We have previously demonstrated that E2 temporally regulates uterine Bip (39, 40), we thus analyzed whether kepone-dependent regulation of Bip mRNAs occurs in a temporal fashion. Consistent to our previous studies, E2 was able to induce uterine Bip mRNAs early (within 2 h) and maintained high until 24 h (Fig. 2G). In case of kepone, as shown in Fig. 2E, our results indicate that kepone at its lowest dose (7.5 mg/kg) had an induction of Bip mRNAs by 2 h (~ 3 fold), over the control. However, this was declined to the basal level by 6h and remained low until 48 h. In contrast, the applications of kepone using doses 15 (data not shown) and 30 mg/kg (Fig. 2F) were able to show the increase of Bip mRNAs by 2h (~ 2 fold), followed by steady increases at 6 and 24 h (4 to 5 fold), and thereafter slight decline by 48 h. Overall, these results suggest that kepone maintains sustained upregulation of uterine Bip RNAs only at or above 15 mg/kg in mice.
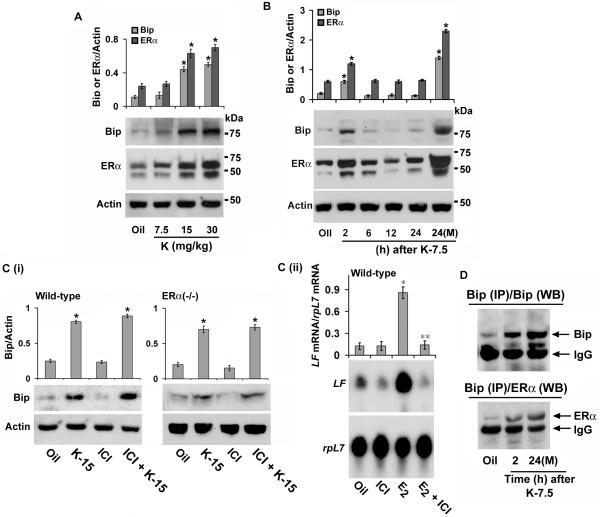
To determine whether this effect of kepone is reflected at the protein levels, we also analyzed expression by Western blotting. Consistent to the above, the analysis of dose-dependent effects of kepone by 24 h revealed a significant induction of uterine Bip levels (~ 4 fold) at doses 15 and 30 mg/kg (Fig. 3A). While, kepone at 7.5 mg/kg was not effective, however, the analysis of the same dose of kepone at different times exhibited an increase of Bip levels by 2 h (~ 3 fold) but failed to sustain during the observed periods for 24 h (Fig. 3B). These results are also consistent with the mRNA data. In contrast, the multiple injections (×3) of kepone (7.5 mg/kg) demonstrated a distinct upregulation of Bip (~ 6 fold) by 24 h of the last injection (Fig. 3B), suggesting a cumulative effect on gene expression after the chronic exposure of kepone. Because Bip controls estrogen regulated ERα functions (40), we also analyzed the expression of ERα protein levels in the above conditions. Concomitant changes were revealed by kepone between the ERα and Bip levels in the mouse uterus (Figs. 3A and B).
Fig. 3. Regulation of Bip in the uteri of wild-type and ERα(−/−) mice by kepone.
A, Dose response studies. Adult ovariectomized wild-type mice were injected (sc) with kepone at 7.5, 15, 30 mg/kg, and killed at 24 h. Uterine tissue extracts (30 μg protein) were analyzed by Western blotting for Bip, ERα and Actin. B, Temporal studies. Adult ovariectomized wild-type mice were given a single injection (sc) of kepone at 7.5 mg/kg and analyzed at indicated times. Additionally, mice were injected with multiple (3×) injections of kepone at 7.5 mg/kg and killed 24 h after the last injection [24(M)]. Uterine tissue extracts (30 μg protein) were analyzed by Western blotting for Bip, ERα and Actin. C, (i) Western blot analysis. Regulation of Bip by kepone in the uteri of wild-type and ERα(−/−) mice. Adult ovariectomized wild-type and ERα(−/−) mice were given injections (sc) of oil, kepone (15 mg/kg), ICI (20 mg/kg) or the same dose of ICI 30 min prior to kepone, and mice were killed at 24 h. Uterine tissue extracts (20 μg protein) were analyzed by Western blotting for Bip and Actin. C, (ii) Northern blot analysis. ICI effectively blocks uterine LF regulation by E2 in the uteri of wild-type mice. Adult ovariectomized wild-type mice were given injections (sc) of oil, E2 (4 μg/mouse), ICI (20 mg/kg) or the same dose of ICI 30 min prior to E2, and mice were killed at 24 h. In general, 3-5 mice were used for each group in above analyses (A-C). These experiments were repeated three times with independent samples. In the bar plot, the abundance of each protein expression was quantitated by analysis of band intensities using densitometric scanning and was corrected against Actin. *Values are statistically different (p<0.05, student’s t test) from the corresponding control group. **Values are statistically different (p<0.05, student’s t test) from the E2-treated group. D, Analysis of protein-protein interaction. Uterine tissue extracts for the indicated groups were subjected to immunoprecipitation using Bip antibody, followed by Western blotting using specific antibodies to Bip (upper panel) or ERα (lower panel). The intense bands represent heavy chain subunit of IgG (this also serves as an internal loading control). In our control experiments, immunoprecipitation using normal goat serum did not detect any specific bands for Bip or ERα by Western blotting (data not shown). These experiments were repeated two times with similar results.
Kepone regulates Bip via ER-independent mechanism, but directs interaction between Bip and ERα in the mouse uterus
Previously we have shown that E2-dependent regulation of uterine Bip is mediated via ER-independent mechanism (39), we thus wanted to examine whether kepone-induced uterine Bip response follows a similar pathway of action. Based on above results, ovariectomized wild-type and ERα(−/−) mice were similarly subjected to kepone (15 mg/kg) or oil, and analyzed uterine levels of Bip by Western blotting experiments. Our results revealed that upregulation of uterine Bip (~ 3.5 −4.0 fold) was indeed detected by kepone over the oil control in both mice [Fig. 3C (i)]. Furthermore, an injection of ER-antagonist ICI 182,780 (ICI, 20 mg/kg) given 30 min prior to kepone was not antagonistic to the above response, while ICI by itself had very little effects [Fig. 3C (i)]. Moreover, consistent to the previous report (20, 21), this dose of ICI was strongly inhibitory to E2-dependent regulation of uterine lactoferrin (LF) gene expression in ovariectomized wild-type mice [Fig. 3C (ii)]. Overall, these results suggest that kepone-dependent regulation of uterine Bip is mediated via ER-independent mechanism.
Because, Bip’s role is primarily mediated through protein-protein interaction and because Bip molecularly interacts with ERα in order to control ERα-dependent uterine estrogen signaling that includes uterine growth and gene expression (40), we wanted to examine whether kepone-induced uterine Bip also demonstrates a similar protein-protein interaction with ERα. Uterine protein extracts were subjected to co-immunoprecipitation analysis using either Bip (Fig. 3D) or ERα (data not shown) antibodies, followed by Western blotting for each of these proteins. While, low level of interaction between Bip and ERα was detected in the oil group, a markedly induced interaction between these proteins was revealed by kepone treatments (Fig 3D). Furthermore, this interaction appears to be specific, since Bip specific antibody was unable to pull down PR (data not shown), a kepone responsive gene in the mouse uterus (21). Additionally, immunoprecipitation using preimmune serum did not detect any specific bands for Bip and ERα by Western blotting (data not shown). Collectively, these results suggest that kepone influences uterine Bip without involving ERs, but directs facilitated interaction of Bip with ERα.
Uterotrophic effect of Kepone strongly correlates with the uterine levels of Bip and this effect is critically dependent on ERα
Because Bip essentially controls estrogen-dependent ERα-mediated uterine growth (40), we examined whether kepone-dependent upregulation of uterine Bip correlates with the ERα-dependent uterotrophic changes in mice. Similar to the E2 effects (Fig. 4A), ovariectomized wild-type mice, given injections of kepone either a single at 15 or 30 mg/kg, or multiple (X3) at 7.5 mg/kg and analyzed 24 h after the last injection, were responsive to significant increases in uterine wet weights, as compared to oil (Fig. 4A). While, a single injection of kepone at 7.5 mg/kg was not effective (Fig. 4A). Furthermore, analysis of uterine cell proliferation, using BrdU incorporation (Fig. 4B and C) and Ki-67 immunostaining (Fig. 4C), revealed a similar results. Interestingly, these observations were consistent with the detection of heightened levels of uterine Bip by kepone at higher doses (15-30 mg/kg) (for comparison see Figs. 3A and B).
Fig. 4.
A, Analysis of uterine wet weights. Adult ovariectomized wild-type mice (n = 10-12 mice in each group) were given single injections of oil, E2 (4 μg/kg), kepone (K, at 7.5, 15 or 30 mg/kg) or multiple injections of K [3X at 7.5 mg/kg, K-7.5 (M)], and killed 24 h after the last injection. In addition, ovariectomized ERα(−/−) mice (n = 5-7 mice in each group) were subjected to kepone (30 mg/kg) and oil, and killed at 24 h. *Values are statistically different (p<0.001), based on ANOVA (F = 99.7, df = 29) followed by Dunnett t-test. B, BrdU incorporation into DNA. Similar to the above experiments in A, mice were injected with BrdU (50 mg/kg, ip) 2h before killing. Formaldehyde fixed paraffin-embedded tissue sections were stained for BrdU incorporation as described in the Materials and Methods. Representative data are shown from the analysis of at least 5 different mice for each group. tissue sections are shown. Reddish-brown nuclear deposits (as shown by dark black spots in the nuclei of epithelial cells) indicate sites of positive immunostaining. le, luminal epithelium; ge, glandular epithelium. C, Quantitation of BrdU- or Ki-67-positive cells. The data presented here after the analysis of at least 5 different mice from each group. *Values are statistically different (p<0.001) against the oil (control), based on ANOVA (BrdU: F = 1782, df = 29; Ki-67: F = 1755, df = 29) followed by Dunnett t-test. D, Analysis of uterine cell proliferation by BrdU or Ki-67 in ERα(−/−) mice. Adult ovariectomized ERα(−/−) mice were given injections (sc) of oil or kepone (30 mg/kg) and analyzed after 24 h. Representative data are shown from the analysis of at least 5 different mice for each group. le, luminal epithelium.
We also examined whether this kepone-dependent regulation of cell proliferation is critical to the ERα, using ERα(−/−) mice. Since above studies demonstrated that kepone at 30 mg/kg is responsive to uterotrophic changes in wild-type mice, ERα(−/−) mice were subjected in a similar manner, and uterine wet weight and cell proliferation were analyzed. Our results revealed that ERα(−/−) mice were unable to support uterine growth responses by kepone (Fig. 4A and D), although this kepone treatment did show distinct upregulation of uterine Bip [Fig. 3C(i)]. Overall, these results, in conjunction with the above protein-protein interaction studies, suggest that kepone-dependent uterotrophic effect is critically dependent on the cooperation of Bip and ERα.
Adenovirus-driven manipulation of uterine Bip modifies estrogenic potency of kepone
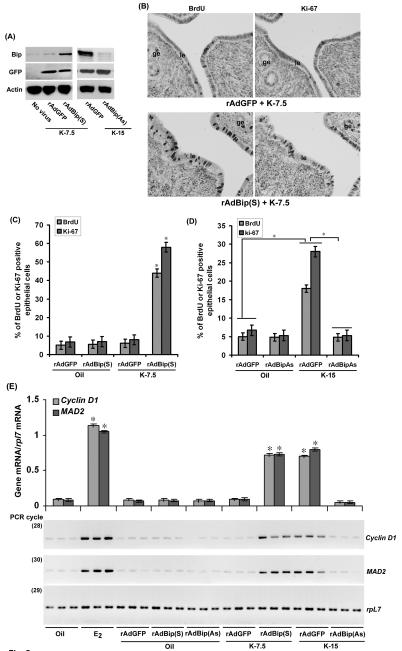
The above results suggested that kepone-induced uterine cell proliferation associates with the heightened levels of endogenous Bip. Since Bip can regulate E2-depedent uterine cell proliferation (40) and since a single injection of kepone at 7.5 mg/kg failed to induce Bip, we speculated that forced expression of Bip by adenovirus-driven approach will accentuate cell proliferation using this sub-optimal dose of kepone. To address this possibility, we first analyzed the status of uterine Bip levels after administration of adenoviruses [rAdGFP (control) or rAdBip(S) or rAdBip(AS)]. As shown in Fig. 5A, Western blot analyses show that delivery of rAdBip(S) virus was indeed effective to maintain forced expression of Bip in the uterus, as compared to the control virus. In contrast, rAdBip(As) was able to suppress kepone-induced uterine Bip level (Fig. 5A). Overall, these results suggest that adenovirus-driven manipulation of Bip expression in the mouse uterus is effective. Under this condition of Bip overexpression, we also analyzed the status of uterine cell proliferation in the wild-type mice, given a sub-optimal dose of kepone (7.5 mg/kg). As shown by BrdU incorporation and Ki-67 immunostaining (Figs. 5B and C), our results demonstrate that forced expression of Bip [via rAdBip(S)] in conjunction with kepone (7.5 mg/kg) was indeed supportive of uterine cell proliferation (7 fold), while the control virus was not effective. Moreover, either of these viral treatments did not show any growth regulatory response in mice when injected with oil (Fig. 5C).
Fig. 5.
A, Analysis of adenovirus mediated expression in the uterus. Uterine tissues were collected after administration of adenoviruses rAdBip(S), rAdBip(As) or rAdGFP (control) in mice as described in materials and methods, followed by injections of kepone as indicated for 24 h. Proteins were analyzed by Western blotting for the expression of Bip, GFP and actin. This experiment was repeated at least three times with similar results. B, Analysis of uterine cell proliferation by BrdU or Ki-67. Adult ovariectomized wild-type mice were subjected to adenoviruses rAdBip(S) or rAdGFP (as control) as described in the Materials and Methods, followed by injections (sc) of kepone (7.5 mg/kg) for 24 h. BrdU (50 mg/kg, ip) was injected 2h before killing. Representative tissue sections are shown from the analysis of at least 5 different mice for each group. le, luminal epithelium, ge, glandular epithelium. C, D, Quantitation of BrdU- or Ki-67-positive cells. The data presented here after the analysis of at least 5 different mice from each group. *Values are statistically different (p<0.001), based on ANOVA (For Fig. 5C: F = 2368, df = 19 for BrdU; F = 1099, df = 19 for Ki-67 and for Fig. 5D: F = 1203, df = 19 for BrdU; F = 1532, df = 19 for Ki-67) followed by Dunnett t-test. E, Kepone regulates uterine expression of cyclin D1 and Mad2 in wild-type mice. Uterine tissues were collected after administration of adenoviruses rAdBip(S), rAdBip(As) or rAdGFP (control) in mice as described in materials and methods, followed by injections of kepone as indicated for 24 h. In addition, adult ovariectomized wild-type mice were subjected to oil (vehicle control) or E2 (100 ng/mouse, as positive control) and killed after 24 h. In general, 3-5 mice were used for each group analysis. Independent preparation of total RNAs (in triplicate) were analyzed by comparative RT-PCR as described in the materials and methods. The band intensities shown in the figure were measured by densitometric analysis and the relative levels of mRNAs for gene specific expression were determined after correction with that of rpL7. *Values are statistically different (p<0.05, Student’s t test) compared to corresponding groups.
To examine further whether Bip is specifically involved in the regulation of kepone-induced uterine cell proliferation, we introduced rAdBip(As) or rAdGFP (as control) in mice prior to the administration of kepone (15 mg/kg) or oil (as vehicle control). As shown before, kepone at this dose, as compared to oil, was effective in inducing cell proliferation in presence of the control virus (Fig. 5D). While, knock-down of Bip via rAdBip(As) showed strong suppression of this kepone-induced effect (Fig. 5D).
Because adenovirus-driven manipulation of Bip affects kepone-induced ERα-dependent uterine cell proliferation, we have further characterized the regulation of expression of several growth regulatory estrogen-responsive ER-mediated uterine genes (cyclin D1 and Mad2) in this context (50). Consistent to the above analysis, our results revealed that either forced expression or suppression of Bip caused respective increase or decrease of ERα-dependent gene expression by kepone as compared to appropriate controls (Fig. 5E). Collectively, these results suggest that overexpression or suppression of Bip strongly correlates with respective increase or decrease of cell proliferation and gene expression in the uteri of mice, in conjunction with kepone.
Stress-regulated signals modulate estrogenicity of kepone in mice
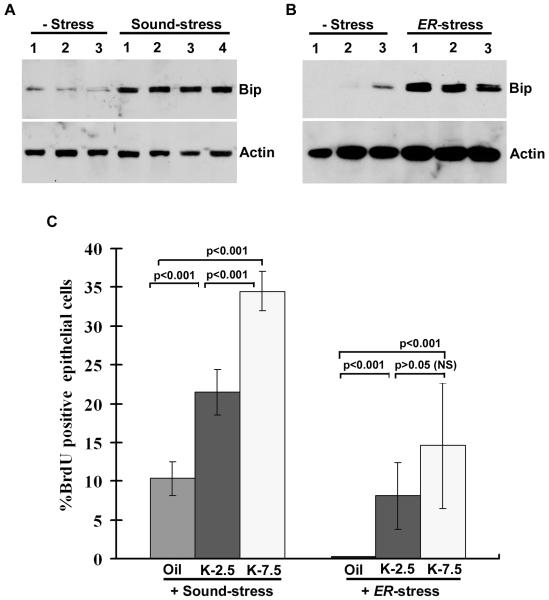
Because Bip is known to be induced by variety of stress signals (42), and because increased uterine levels of Bip enhance estrogenicity of kepone, we speculate that stress inducing signals might enhance growth responsive effects by sub-lethal doses of kepone. As described in the Materials and Methods, ovariectomized wild-type mice were subjected to kepone (2.5 and 7.5 mg/kg), in combination with different stress responses. Previously, it was shown that ultrasonic sound stress or endoplasmic reticulum (ER) stress, known to induce stress-specific signals in tissues of mice (51, 52). In our initial analysis we wanted to examine whether these stress inducers alter the levels of uterine Bip. As shown in Fig. 6A-B, we indeed observed a dramatic induction of uterine Bip in mice, subjected to either stress. Next, we wanted to examine whether stress influences uterine cell proliferation in mice, exposed to sub-lethal doses of kepone (2.5 and 7.5 mg/kg) or oil (as vehicle control). As shown in Fig. 6C, both stress inducers were able to support increased uterine cell proliferation in presence of either doses of kepone, as compared to oil, although kepone-dependent alterations in the ER-stress group were lower in effect, suggesting a negative influence appeared to be mediated by tunicamycin. Furthermore, our statistical analysis shows that dose dependent increases of uterine cell proliferation by kepone in the ER-stress group is not significant, although individually they are significant against the oil (Fig. 6C). Overall, these results suggest that stress-inducing signals influence kepone-dependent growth regulation, presumably via alteration of uterine Bip.
Fig. 6.
Analysis of uterine Bip and Actin expression by Western blotting in mice (n = 3-4 mice), following the ultrasonic sound stress (A) or ER-stress by injection of tunicamycin (1.0 mg/kg, sc) (B). C, Quantitative analysis of BrdU incorporation. Adult ovariectomized wild-type mice were subjected to stress either by utltrasonic sound or tunicamycin (1.0 mg/kg, sc) injection, and analyzed after 24 h. Statistical significance was performed using ANOVA (F = 87.4, df = 11 for sound-stress studies; F= 7.6, df = 11 for ER-stress studies), followed by Dunnett t-test. NS is not significant.
Discussion
Although kepone-induced actions in the uterus have been implicated estrogenic, the molecular mechanisms of this action remain poorly understood. In this regard, the highlight of the present investigation is that the degree of uterine estrogenicity by kepone can be potentially amplified by an endoplasmic reticulum protein Bip in the mouse uterus. The application of adenovirus-driven strategy using rAd-Bip(S) construct or stress-mediated signals appeared to cause sustained upregulation of uterine Bip, which also resulted in susceptible increase in uterine epithelial cell proliferation in mice, given sub-optimal dose of kepone. This finding is highly significant with regard to the reproductive toxicity attributed to a large variety of polychlorinated hydrocarbons that still persists in the environment (1, 4-6). Based on existing literatures (21, 22, 53-56), it has been clearly shown that doses of the tested xenoestrogenic compounds used in the present study exhibit estrogen-like activities in rodent uterus viz. wet weights, epithelial thickness and expressional regulation of estrogen-responsive genes. The basis for reproductive toxicity of these compounds was thought to be mediated by their interactions with the nuclear ERs of the target tissues. Consistent to this notion, our studies provide evidence that kepone essentially utilizes ERα in controlling the uterine growth response in mice. Furthermore, in conjunction with our previous studies (40), it is now evident to propose that E2 and kepone could elicit uterine Bip, which then mediates a cross-talk with ERα via protein-protein interaction in order to control ERα-mediated nuclear gene transcription that is considered to be fundamental for manifestation of full complement of estrogenic activity in the uterus.
Recently, Bip and Wnt-signaling genes have been identified as non-classical targets, since they do not involve nuclear ERs during the regulation by E2 or 4OH-E2 in the uteri of mice (39, 44). In the present study, we wanted to examine whether kepone, methoxychlor and o,p’-DDT exhibit any non-classical responses to these gene signaling systems. Our analysis reveled that kepone selectively modulates uterine Bip mRNAs, without influencing Wnt-signaling genes, primarily through dose and time-dependent manners (Figs. 2 and 3). In contrast, methoxychlor and o,p’-DDT were totally ineffective to these non-classical gene targets (Fig. 2). Our observations with distinct regulation of uterine gene by estrogenic compounds is not surprising. Indeed. it has been reported that kepone and 4-OH-E2 were able to induce LF gene in the uteri of ERα null mice, while E2 was completely ineffective, suggesting estrogenic compounds can have differential effects on uterine genes (20).
Accumulating evidence suggests that natural and environmental estrogens modulate uterine genes without involving ERs (20-22, 38, 39, 44). These studies primarily based on the observations that ERα null or wild-type mice, in which ER functions are silenced by ER antagonist ICI 182,780 (ICI) manifest expression of uterine genes in response to estrogenic compounds. Consistently, our findings of up-regulation of uterine Bip by kepone in wild-type and ERα(−/−) mice [Fig. 3C(i)] in presence or absence of ICI suggest a similar ER-independent mechanism is operative for kepone-dependent regulation of uterine Bip in mice.
Our findings of uterine regulation of Bip expression and the increase in uterine wet weight and cell proliferation (Figs. 4A-C) requiring concentrations (15-30 mg/kg) or multiple injections (3×) of kepone (7.5 mg/kg) in mice are consistent with the existing literatures (13, 20-22). Moreover, consistent to our observations in the present study, multiple injections of kepone at 7.5 mg/kg have been shown to exhibit additive effects on uterine LF gene expression (20). However, to our knowledge there is no report to indicate that multiple injections of kepone at this dose can cause bioaccumulation of this xenoestrogen in the body. Moreover, one may raise concern about the toxicity of kepone in the body, since this compound known to affect liver and kidney detoxification systems, which may indirectly affect hormone metabolism. However, to our knowledge, there is no evidence to suggest that estrogenic effects (viz., such as DNA and protein synthesis, cell proliferation, uterine weight increase, etc.) of this compound using the doses in the present study in mice, are due to an induced cytotoxicity through other secondary organs. Furthermore, it should also be recognized that we have compared the effects of kepone in the wild-type and ERα(−/−) mice. Thus, the differences as observed by kepone in respect of uterine growth between these mice (Fig. 4), should not be considered as non-specific effects, since such effects should also be seen in both mice.
Bip, as a molecular chaperone, primarily functions to direct appropriate protein folding and assembly and intracellular trafficking (42). Studies have shown that Bip is an abundant protein during the growth regulatory conditions in both normal and tumors tissues (57, 58). In the present study, because Bip was not regulatory by o,p-DDT and methoxychlor in the mouse uterus (Fig. 2B and C), and because uterine Bip is specifically altered by kepone, our further studies of Bip in conjunction with uterine growth, precludes studies involving estrogenicity of o,p’-DDT and methoxychlor. Previously we have shown that Bip plays an essential role via protein-protein interaction, to control nuclear ERα functions in respect of gene transcription and growth regulation by E2 (40). Consistent to these results, we observed that kepone regulates uterine Bip, in absence of ERα [Fig. 3C(i)]. Additionally, Bip molecularly interacts with ERα under the direction of kepone (Fig. 3D), suggesting that Bip may regulate kepone-dependent ERα function. This was strongly supported by our observation that kepone-dependent ERα-mediated gene expression is abrogated after suppression of uterine Bip via adenovirus approaches (Fig. 5E). Furthermore, it was observed that regulation of uterine Bip is closely followed by uterine growth in presence of kepone in the wild-type mice (Fig 3A,B vs. Fig. 4A-C) and this is again strongly compromised after knock-down of uterine Bip expression (Fig. 5D), suggesting uterine Bip is critically involved in this regulation. In contrast, studies also showed that upregulation of uterine Bip did not correlate with the growth response in presence of kepone in ERα(−/−) mice [Fig. 3C(i) vs. Fig. 4A,D], suggesting that Bip must cooperate with ERα in order to have uterine growth under the direction of kepone. This result is further consistent with the observation that forced expression of uterine Bip in the wild-type mice did not lead to the cell proliferation in presence of oil. Overall these results suggest that kepone-dependent uterine growth response utilized a molecular cross-talk between Bip and ERα.
The major thrust of this work was to demonstrate that sustained levels of uterine Bip can be detrimental to kepone’s action in mice. This idea was primarily evolved from our previous report that Bip is regulatory for estrogen-dependent ERα-mediated uterine growth (40) and from our present findings that kepone at a sub-lethal dose 7.5 mg/kg is unable to sustain upregulation of uterine Bip expression (Figs. 2 and 3) and cell proliferation (Fig. 4) in mice. This was further supported by our observations of ERα-dependent enhanced uterine cell proliferation and gene expression in mice, after exposure to sub-lethal dose of kepone, in combination with forced expression of uterine Bip via adenovirus-driven strategy (Fig. 5B,C and E). Overall, our studies, using both sense or antisense adenoviruses for Bip, suggest that heightened expression of Bip is strongly correlated with the ability of kepone to induce ERα-dependent uterine cell proliferation and gene expression (Fig. 5B-E). In this regard, it should be noted that a similar sort of enhancement of ligand-dependent sensitivity for nuclear receptor functional activities has been reported for some xenobiotic compounds (59).
It is known that the regulation of cellular Bip occurs under a variety of conditions, including stress, chemical toxicity, treatment with Ca2+ ionophores and inhibitors of glycosylation that all influence endoplasmic reticulum (ER) (42). Furthermore, it has been shown that the effects of chronic stress cause enhanced uterine growth response by E2 in rats (60), therefore, we speculated that stress-induced uterine Bip may support uterine estrogenicity for a sub-optimal dose of kepone. Consistent to this speculation, indeed our observations showed that the increase of uterine cell proliferation occurs in correlation with heightened levels of uterine Bip in mice that were subjected to stress in combination with kepone at sub-optimal doses (2.5 or 7.5 mg/kg) (Fig. 6). While, in general, less responsive effects for cell proliferation in the ER-stress group appear to suggest that tunicamycin may impose an inhibition of cell proliferation, as implicated in the literature (61, 62). Overall, these results revealed that endogenous Bip via stress-related signals contributes to the establishment of uterine estrogenicity for kepone.
In summary, studies provide a novel evidence that Bip can be considered as a major regulator to amplify estrogenic potency for a weak xenoestrogen. Furthermore, since Bip is regulatory by variety of signals in the body, including stress and cancer, these may thus act as plausible risk factors to produce enhanced estrogenicity for xenoestrogen, which should be a major health concern.
Acknowledgements
This work was supported in parts by the National Institutes of Health (NIH) Grants (HD37830, ES07814).
Footnotes
Disclosures
The authors have nothing to disclose.
Publisher's Disclaimer: NIH statement: This is an un-copyedited author manuscript copyrighted by The 13 Endocrine Society. This may not be duplicated or reproduced, other than for personal use or 14 within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without 15 permission of the copyright owner, The Endocrine Society. From the time of acceptance 16 following peer review, the full text of this manuscript is made freely available by The Endocrine 17 Society at http://www.endojournals.org/. The final copy edited article can be found at 18 http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for 19 errors or omissions in this version of the manuscript or in any version derived from it by the 20 National Institutes of Health or other parties.”
References
- 1.Simonich SL, Hites RA. Global distribution of persistent organochlorine compounds. Science. 1995;269:1851–1854. doi: 10.1126/science.7569923. [DOI] [PubMed] [Google Scholar]
- 2.Schapira A. DDT: a polluted debate in malaria control. Lancet. 2006;368:2111–2113. doi: 10.1016/S0140-6736(06)69812-7. [DOI] [PubMed] [Google Scholar]
- 3.Gunasekaran K, Sahu SS, Jambulingam P, Das PK. DDT indoor residual spray, still an effective tool to control Anopheles fluviatilis-transmitted Plasmodium falciparum malaria in India. Trop Med Int Health. 2005;10:160–168. doi: 10.1111/j.1365-3156.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 4.Manz M, Wenzel KD, Dietze U, Schuurmann G. Persistent organic pollutants in agricultural soils of central Germany. Sci Total Environ. 2001;277:187–198. doi: 10.1016/s0048-9697(00)00877-9. [DOI] [PubMed] [Google Scholar]
- 5.Backe C, Cousins IT, Larsson P. PCB in soils and estimated soil-air exchange fluxes of selected PCB congeners in the south of Sweden. Environ Pollut. 2004;128:59–72. doi: 10.1016/j.envpol.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Wang C, Wang Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ Int. 2005;31:778–783. doi: 10.1016/j.envint.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Lu Y, Dawson RW, Shi Y, Wang T. Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere. 2005;60:762–769. doi: 10.1016/j.chemosphere.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante MC, Cirillo T, Naso B, Clausi MT, Lucisano A, Cocchieri RA. Polychlorinated biphenyls and organochlorine pesticides in seafood from the Gulf of Naples (Italy) J Food Prot. 2007;70:706–715. doi: 10.4315/0362-028x-70.3.706. [DOI] [PubMed] [Google Scholar]
- 9.Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, Saville B, McDonnell D. Toxicology of environmental estrogens. Reprod Fertil Dev. 2001;13:307–315. doi: 10.1071/rd00108. [DOI] [PubMed] [Google Scholar]
- 10.Singleton DW, Khan SA. Xenoestrogen exposure and mechanisms of endocrine disruption. Front Biosci. 2003;8:s110–118. doi: 10.2741/1010. [DOI] [PubMed] [Google Scholar]
- 11.Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- 12.Wolff MS, Toniolo P, Lee E, Rivera M, Dubin N. Blood levels of organochlorine residues and the risk of breast cancer. J Natl Can Inst. 1993;85:648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Paria BC, Johnson DC, Dey SK. Embryo-Uterine Interactions During Implantation: Potential Sites of Interference by Environmental Toxins. In: Sipes G, McQueen CA, Gandolfi AJ, editors. Comprehensive Toxicology, Volume 10, Reproductive and Endocrine Toxicology. Elsevier Science Ltd.; New York: 1997. pp. 317–328. [Google Scholar]
- 14.Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. Medical hypothesis: Xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993;101:372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safe S. Environmental and dietary estrogens and human health: Is there a problem. Environ Health Perspect. 1995;103:346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 19.Safe S, Connor K, Ramamoorthy K, Gaido K, Maness S. Human Exposure to Endocrine-Active Chemicals: Hazard Assessment Problems. Regul Toxicol Pharmacol. 1997;26:52–58. doi: 10.1006/rtph.1997.1118. [DOI] [PubMed] [Google Scholar]
- 20.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh D, Taylor JA, Green JA, Lubahn DB. Methoxychlor stimulates estrogen-responsive messenger ribonucleic acids in mouse uterus through a non-estrogen receptor (non-ER) alpha and non-ER beta mechanism. Endocrinology. 1999;140:3526–3533. doi: 10.1210/endo.140.8.6877. [DOI] [PubMed] [Google Scholar]
- 23.Simons SS, Jr, Oshima H, Szapary D. Higher levels of control: modulation of steroid hormone-regulated gene transcription. Mol Endocrinol. 1992;6:995–1002. doi: 10.1210/mend.6.7.1324423. [DOI] [PubMed] [Google Scholar]
- 24.Kupfer D, Bulger WH. Inactivation of the uterine estrogen receptor binding of estradiol during P-450 catalyzed metabolism of chlorotrianisene (TACE). Speculation that TACE antiestrogenic activity involves covalent binding to the estrogen receptor. FEBS Lett. 1990;261:59–62. doi: 10.1016/0014-5793(90)80636-w. [DOI] [PubMed] [Google Scholar]
- 25.Meyers CY, Kolb VM, Gass GH, Rao BR, Roos CR, Dandliker WB. Doisynolic-type acids: uterotrophically potent estrogens which compete poorly with estradiol for cytosolic estradiol receptors. J Steroid Biochem. 1988;31:393–404. doi: 10.1016/0022-4731(88)90307-x. [DOI] [PubMed] [Google Scholar]
- 26.Meyers CY, Lutfi HG, Adler S. Transcriptional regulation of estrogen-responsive genes by non-steroidal estrogens: doisynolic and allenolic acids. J Steroid Biochem Mol Biol. 1997;62:477–489. doi: 10.1016/s0960-0760(97)00063-0. [DOI] [PubMed] [Google Scholar]
- 27.Bulger WH, Muccitelli RM, Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27:2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- 28.Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- 29.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (rba-sma) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octyphenol. Environ Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan DM, Willingham E, Gaylor D, Bergeron JM, Crews D. No threshold dose for oestradiol-induced sex reversal of turtle embryos: How little is too much? Environ Health Perspect. 1999;107:155–159. doi: 10.1289/ehp.99107155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa J, Saito K, Goto J, Dakeyama F, Matsuo M, Nishihara T. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol Appl Pharmacol. 1999;154:76–83. doi: 10.1006/taap.1998.8557. [DOI] [PubMed] [Google Scholar]
- 34.Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- 35.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 38.Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147:S25–32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- 39.Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. Estrogen targets genes involved in protein processing, calcium homeostasis and Wnt signaling in the mouse uterus independent of estrogen receptor-α and -β. J Biol Chem. 2000;275:28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- 40.Ray S, Hou X, Zhou HE, Wang H, Das SK. Bip is a molecular link between the phase-I and phase-II estrogenic responses in uterus. Mol Endocrinol. 2006;20:1825–1837. doi: 10.1210/me.2006-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 43.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11186. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen mediated uterine growth. Mol Endocrinol. 2004;18:3005–3044. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das SK, Chakraborty I, Paria BC, Wang X-N, Plowman GD, Dey SK. Amphiregulin is a implantation specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–701. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 46.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290:105–117. doi: 10.1016/j.ydbio.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FKBP52 as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- 50.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 51.Tometten M, Blois S, Kuhlmei A, Stretz A, Klapp BF, Arck PC. Nerve growth factor translates stress response and subsequent murine abortion via adhesion molecule-dependent pathways. Biol Reprod. 2006;74:674–683. doi: 10.1095/biolreprod.105.044651. [DOI] [PubMed] [Google Scholar]
- 52.Kondoh M, Tsukada M, Kuronaga M, Higashimoto M, Takiguchi M, Himeno S, Watanabe Y, Sato M. Induction of hepatic metallothionein synthesis by endoplasmic reticulum stress in mice. Toxicol Lett. 2004;148:133–139. doi: 10.1016/j.toxlet.2003.12.066. [DOI] [PubMed] [Google Scholar]
- 53.Welch RM, Levin W, Conney AH. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969;14:358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]
- 54.Eroschenko VP, Rourke AW, Sims WF. Estradiol or methoxychlor stimulates estrogen receptor (ER) expression in uteri. Reprod Toxicol. 1996;10:265–271. doi: 10.1016/0890-6238(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 55.Bulger WH, Kupfer D. In: Endocrine Toxicology. Thomas JA, Korach KS, McLachlan JA, editors. Raven Press; New York: 1985. pp. 1–33. [Google Scholar]
- 56.Hammond B, Katzenellenbogen BS, Krauthammer N, McConnell J. Estrogenic activity of the insecticide chlordecone (Kepone) and interaction with uterine estrogen receptors. Proc Natl Acad Sci U S A. 1979;76:6641–6645. doi: 10.1073/pnas.76.12.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons DG, Kennedy TG. Induction of glucose-regulated protein 78 in rat uterine glandular epithelium during uterine sensitization for the decidual cell reaction. Biol Reprod. 2000;62:1168–1176. doi: 10.1095/biolreprod62.5.1168. [DOI] [PubMed] [Google Scholar]
- 58.Lee AS. GRP78 Induction in Cancer: Therapeutic and Prognostic Implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 59.Jansen MS, Nagel SC, Miranda PJ, Lobenhofer EK, Afshari CA, McDonnell DP. Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2004;101:7199–7204. doi: 10.1073/pnas.0402014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunin AG. Effect of chronic stress on estradiol action in the uterus of ovariectomized rats. Eur J Obstet Gynecol Reprod Biol. 1996;66:169–174. doi: 10.1016/0301-2115(96)02329-9. [DOI] [PubMed] [Google Scholar]
- 61.Schraen-Maschke S, Zanetta JP. Role of oligomannosidic N-glycans in the proliferation, adhesion and signalling of C6 glioblastoma cell. Biochimie. 2003;85:219–229. doi: 10.1016/s0300-9084(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 62.Harrison ML, Villemez CL. Protein glycosylation is required for the proliferation of mitogen-activated lymphocytes. J Immunol. 1981;127:2616–2617. [PubMed] [Google Scholar]