Abstract
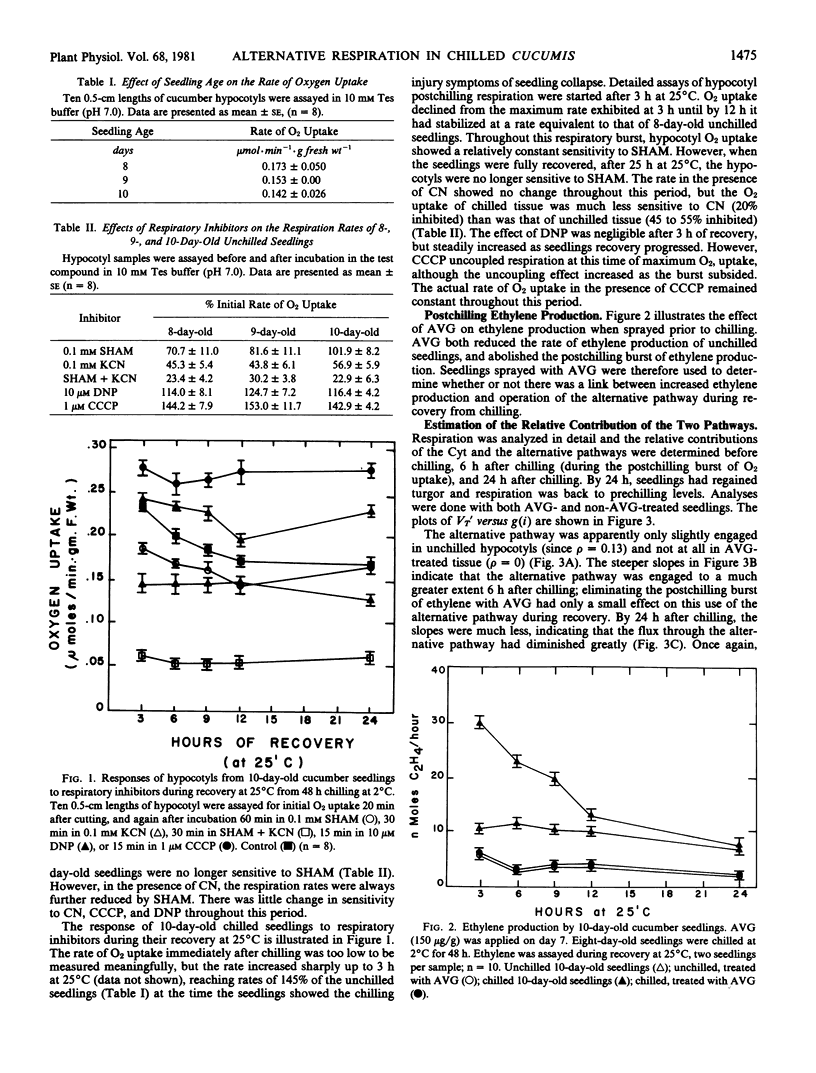
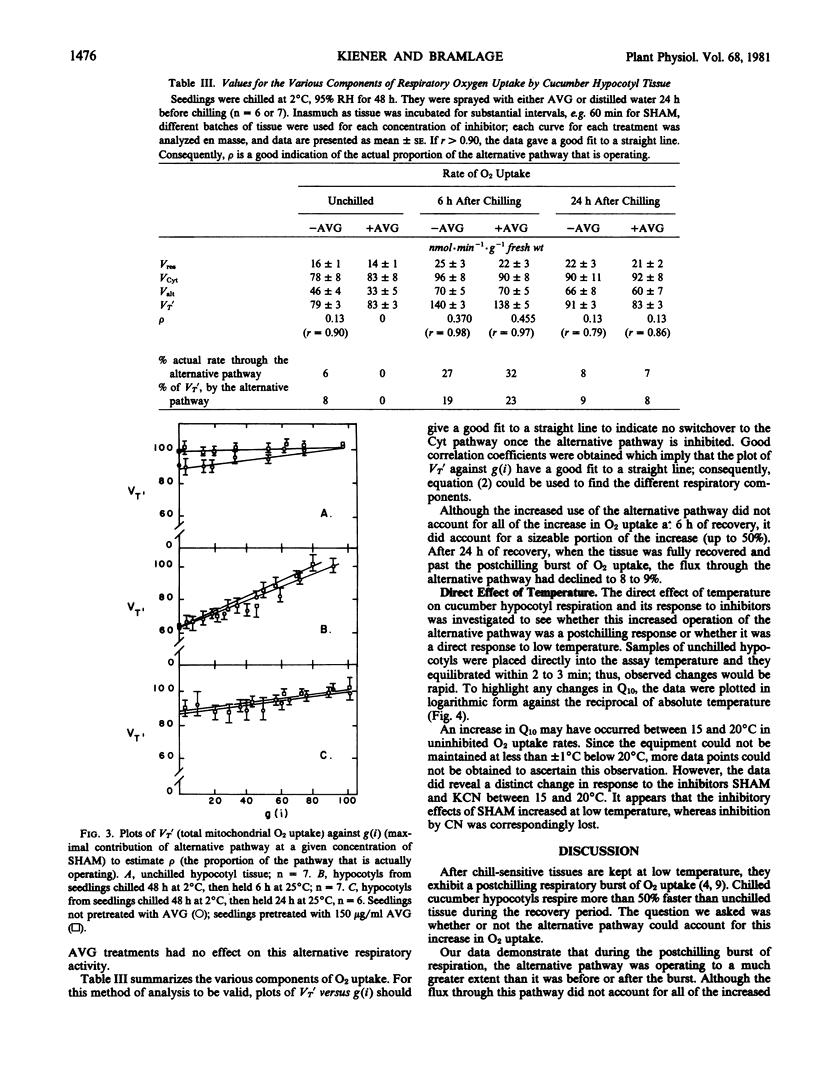
After 48 hours at 2°C, hypocotyls from chill-sensitive Cucumis sativus seedlings showed a burst of O2 uptake. The alternative pathway became engaged to close to 45% full capacity during this postchilling respiratory burst. However, it only accounted for up to 50% of this increased respiratory O2 uptake. By 24 hours after chilling, when the seedlings were fully recovered from visible symptoms of chilling injury, the flux through the alternative pathway was back to the level (about 10%) found before chilling. Blocking chilling-induced ethylene production with aminoethoxyvinylglycine had no effect on this increased utilization of the alternative pathway.
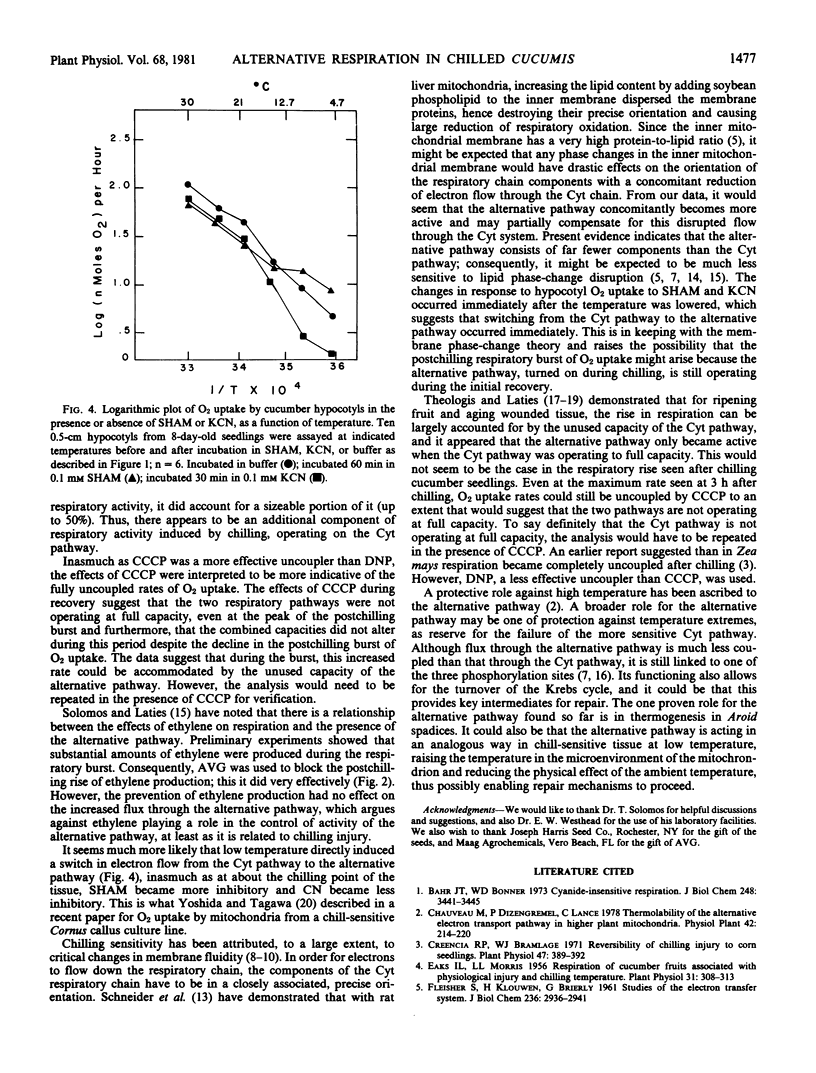
The direct effects of temperature on respiration rates and the effects of inhibitors suggested that there was a rapid increase in alternative pathway activity and decrease in the cytochrome pathway activity. The possibility that the alternative pathway represents a compensatory mechanism for the more labile cytochrome pathway is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Creencia R. P., Bramlage W. J. Reversibility of chilling injury to corn seedlings. Plant Physiol. 1971 Mar;47(3):389–392. doi: 10.1104/pp.47.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaks I. L., Morris L. L. Respiration of Cucumber Fruits Associated with Physiological Injury at Chilling Temperatures. Plant Physiol. 1956 Jul;31(4):308–314. doi: 10.1104/pp.31.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHER S., KLOUWEN H., BRIERLEY G. Studies of the electron transfer system. 38. Lipid composition of purified enzyme preparations derived from beef heart mitochondria. J Biol Chem. 1961 Nov;236:2936–2941. [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Höchli M., Hackenbrock C. R. Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J Biol Chem. 1980 Apr 25;255(8):3748–3756. [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Induction of ethylene of cyanide-resistant respiration. Biochem Biophys Res Commun. 1976 May 17;70(2):663–671. doi: 10.1016/0006-291x(76)91098-6. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Cyanide-resistant Respiration in Fresh and Aged Sweet Potato Slices. Plant Physiol. 1978 Aug;62(2):243–248. doi: 10.1104/pp.62.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Respiratory Contribution of the Alternate Path during Various Stages of Ripening in Avocado and Banana Fruits. Plant Physiol. 1978 Aug;62(2):249–255. doi: 10.1104/pp.62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]



