Abstract
Objective:
Ischemia and reperfusion injury due to tourniquet application during arthroscopy is a well known problem. This study aimed to compare the effects of dexmedetomidine and ketamine on hemodynamic and respiratory variables and on total anti-oxidant status (TAS), total oxidant status (TOS) and malondialdehyde (MDA) as markers of ischemia-reperfusion injury.
Materials and Methods:
This study was approved by a local ethics committee. The study was performed on patients undergoing arthroscopic operation under spinal anesthesia. Thirty patients were randomized into two groups: Group D (dexmedetomidine; n=15) and Group K (Ketamine; n=15). Spinal anesthesia at the L2–4 level was achieved using a 25G spinal needle with hyperbaric bupivacaine at a dose of 12–15 mg in all patients. In Group D, patients were sedated with dexmedetomidine at a dose of 0.3–0.5 μg/kg/h, while Group K received ketamine at a dose of 1–1.5 mg/kg/h. Hemodynamic parameters, oxygen saturation, Ramsey sedation scale (RSS), and TAS, TOS, and MDA levels were recorded.
Results:
Demographic parameters, TAS, TOS and MDA levels were similar between groups. In Group K, the TOS levels after tourniquet removal were significantly lower than at baseline and during the use of the tourniquet. Preoperative hemodynamic and respiratory variables were similar in both groups. Blood pressure values were decreased compared to baseline but these decreases were not statistically significant.
Conclusion:
In patients undergoing arthroscopy under spinal anesthesia, dexmedetomidine had effects similar to ketamine, led to insignificant alterations in hemodynamic and respiratory variables during surgery and had comparable effects on ischemia-reperfusion injury. Thus, we think that dexmedetomidine can be a safe alternative to ketamine as an intraoperative sedative.
Keywords: Dexmedetomidine, Ketamine, Ischemia reperfusion, Oxidative stress
Özet
Amaç:
Artroskopi esnasında uygulanan turnike sonrası iskemi reperfüzyon hasarı geliştiği bilinmektedir. Bu çalışmada sedatif-anksiyolitik amaçla kullanılan dexmedetomidin ve ketamin’in hemodinamik, respiratuar değerler ve iskemi-reperfüzyon hasarını ve oksidatif stres yanıtını gösteren total anti-oksidan status (TAS), total oksidan status (TOS) ve malondialdehit (MDA) ile karşılaştırılmaları planlandı.
Gereç ve Yöntem:
Hastane yerel etik kuruldan onay alındıktan sonra artroskopi uygulanacak hastalar rasgele iki gruba ayrıldılar. Grup D (Dexmedetomidin; n=15) ve Grup K (Ketamin; n=15) olarak iki grup belirlendi. Hastalara L2–4 seviyesinden 25G spinal iğne ile hiperbarik bupivakain (12–15 mg) kullanarak spinal anestezi sağlandıktan sonra sedatif anksiyolitik amaçla Grup D’de dexmedetomidin (0.3–0.5 μgr/kg/saat), Grup K’de ketamin 1–1.5 mg/kg/saat infüzyon olarak verildi. İntraoperatif hemodinamik değerler, Ramsey sedasyon skalası (RSS), SpO2 ve TAS, TOS ve MDA düzeyine bakıldı.
Bulgular:
Demografik veriler ve TAS, TOS ve MDA değerleri her iki grupta benzerdi. Ketamin verilen grupta grup içi değerlendirmede turnike sonrası TOS değerleri giriş ve turnike sırasındaki değerlerden anlamlı olarak düşük bulundu. Peroperatif olarak hemodinamik ve respiratuar parametrelerde gruplar arası fark yoktu. Her iki grupta da kan basınçlarında başlangıç değerlerine göre azalma oldu, ancak bu istatistiksel olarak anlamlı değildi.
Sonuç:
Spinal anestezi altında turnike uygulanan artroskopi hastalarında sedatif olarak dexmedetomidin uygulanmasının ketaminle benzer hemodinamik ve respiratuar ve iskemi-reperfüzyon değerleri gösterdiği ve ketamine güvenli alternatif olabileceği sonucuna varılmıştır.
Introduction
Surgery of the extremities can be performed under tourniquet control to provide a bloodless field, but this technique causes one of the most common forms of skeletal muscle ischemia-reperfusion (I/R) injury. Prolonged ischemia with tourniquet inflation and subsequent reperfusion causes lipid peroxidation, resulting in tissue injury [1]. Lipid peroxidation in the cellular membrane, which is caused by free oxygen radicals (FOR) occurring during I/R, leads to MDA release, which indicates the degree of oxidative stress and tissue injury [2]. Oxidative stress can be defined as an imbalance between oxidants and antioxidants. Excess oxidants and/or a depletion of antioxidants in the organism cause oxidative stress. Plasma total antioxidant status (TAS) level is another well-established marker of oxidative stress, indicating the anti-oxidant defense status of the organism [3].
The role of anesthetic agents in muscular I/R injury has been evaluated in several previous studies [4–7]. In a previously conducted clinical study the authors investigated the effect of ketamine infusion on tourniquet induced I/R injury during arthroscopic knee surgery by measuring MDA and hypoxanthine (HPX) levels. They reported that ketamine protected neurons against I/R-induced lipid peroxidation [4].
Dexmedetomidine, a highly selective alpha-2 receptor agonist, was reported to be effective in protecting against focal ischemia in rabbits [8], cardiac I/R injury in rats [9], and complete forebrain ischemia in rats [10].
In this study, we wanted to compare the effect of anesthesia with dexmedetomidine with ketamine on the levels of total antioxidant status (TAS- as a marker of anti-oxidant defense system), malondialdehyde (MDA) and total oxidant status (TOS- as a marker of oxidative stress) in the plasma of patients undergoing arthroscopic knee surgery requiring a pneumatic tourniquet under spinal anesthesia.
Materials and Methods
After obtaining ethics committee approval and informed patient consent, we studied 30 ASA physical status I or II patients aged between 18 and 65 scheduled for arthroscopic knee surgery requiring a pneumatic tourniquet. Exclusion criteria included the use of any opioid or sedative medications in the week prior to surgery, a history of alcohol or drug abuse, known allergy to any of the drugs used in study or contraindication to spinal anesthesia (e.g., coagulation defects, infection at the puncture site, pre-existing neurological deficits in the lower extremities), and cardiovascular, respiratory, neurological, psychological, hepatic, or renal disease.
Patients were randomized into two groups using computer randomization: Group D (dexmedetomidin, n=15) and Group K (ketamine, n=15). Patients were not premedicated before surgery. After placement of an 18G venous catheter in the operating room, all patients received 500 mL of lactated Ringer’s solution for intravascular volume loading before spinal anesthesia. Electrocardiography, noninvasive blood pressure measurement, and peripheral oxygen saturation (SpO2) were monitored during the study. After obtaining baseline (Base) blood samples, spinal anesthesia was administered from the L2–4 interspinal space levels with 12.5–15 mg of 0.5% hyperbaric bupivacaine using a 25G spinal needle. When a sensory block level at T8–10 was ensured by the pinprick test, sedation was started. Dexmedetomidine (Precedex 100 mcg/2 ml, Abbott Lab, Chicago, IL, USA) was administered at an infusion rate of 0.3–0.5 μg/kg/h in Group D, while ketamine HCL (Ketalar 500 mg/10 cc, Pfızer, İstanbul, Turkey) was administered at an infusion rate of 1–1.5 mg/kg/h in Group K. All study drugs were administered in the same sized and covered infusion sets by an observer who was unaware of the patients’ group and study drugs. The Ramsey Sedation Scale (RSS) was used to measure sedation before and 5, 10, 20, 30, 45, and 60 minutes after administration of sedation (RSS; 1-anxious and agitated, 2-cooperative and tranquil, 3-drowsy but responsive to commands, 4-asleep but responsive to glabellar tap, 5-asleep with a sluggish response to tactile stimulation, and 6-asleep and no response) [11]. After achieving adequate sedation (RSS score 3), the infusion dose was decreased to 0.3 μg/kg/h dexmedetomidine in Group D and 1 mg/kg/h ketamine in Group K (Table 1). The infusion dose was decreased to half or increased to twice the level of anesthetic to keep the Ramsey score at 3. We also evaluated side effects by assessing respiratory depression (respiration rate ≤10 breaths/min) and nausea/vomiting.
Table 1.
Ramsey Sedation Scale (RSS) scores of the groups
| RSS Time (min) | Group D (n=15) Median (range) | Group K (n=15) Median (range) | p value |
|---|---|---|---|
| Baseline | 2 (1–2) | 2 (1–2) | NS |
| 5.min | 2 (2–3) | 2 (1–3) | NS |
| 10.min | 2 (2–4) | 2 (1–4) | NS |
| 20.min | 2 (2–5) | 2 (2–4) | NS |
| 30.min | 2 (2–5) | 2 (1–4) | NS |
| 45.min | 3 (2–5) | 2 (1–5) | NS |
| 60.min | 3 (2–5) | 2 (2–5) | NS |
(D) dexmedetomidine; (K) Ketamine; (NS) nonsignificant
The tourniquet was applied at a pressure approximately twice the systolic arterial blood pressure. Blood samples were obtained by another observer immediately before spinal anesthesia (Base), 1 min before tourniquet release (BTR) and 10 min after tourniquet release (ATR) for the measurement of MDA, TAS and TOS. Heart rate (HR), blood pressure (systolic, diastolic and mean arterial pressure), respiratory rate (RR), and oxygen saturation (SpO2) were recorded simultaneously. All patients received 2 l/min oxygen through a nasal cannula during the operation. The dexmedetomidine or ketamine infusion was stopped at the start of skin closure.
Venous blood samples were taken at pre-determined times into tubes containing separation gels and serum was separated from the cells by centrifugation at 1500 rpm for 10 min. The samples were stored at −80°C until the time of the study. TAS and TOS were measured by Erel’s methods [12]. Erel’s total antioxidant capacity method is based on the bleaching of the characteristic color of a more stable 2,2’-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) radical cation by antioxidants. Erel’s TOS method is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidant species in an acidic medium and the measurement of the ferric ion by xylenol orange. The results were expressed in mmol Trolox equivalent/L and μmol H2O2/L. Plasma MDA levels were measured as described in the literature [13].
The software program SPSS 13.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis. Data are presented as the mean±standard deviation. Comparisons of the two groups with regard to hemodynamic, respiratory, biochemical data and time of tourniquet use were analyzed with the Mann-Whitney U test; the Wilcoxon sign test was used for intragroup comparisons. Gender and side effects were analyzed via the chi-squared test or Fisher’s exact test, as appropriate. A p-value <0.05 were considered to be statistically significant.
Results
There were no significant differences between the groups in demographic data and operative variables such as duration of tourniquet application. These values are shown in Table 2.
Table 2.
The demographic and operational variables of all patients
| Group D (n=15) | Group K (n=15) | |
|---|---|---|
| Gender (M/F) | 11/4 | 13/2 |
| Body weight (kg) | 78.2±14.7 | 72.3±10.3 |
| Length (cm) | 172.4±12.3 | 171.8±6.2 |
| ASA physical status (I/II) | 7/8 | 9/6 |
| Tournique time (min) | 49.8±18.3 | 38.2±14.1 |
(ASA) American Society of Anesthesiologists
Heart rate values changed after sedation compared to baseline values. In group D, the heart rate values after sedation were decreased significantly in all measurement periods compared to baseline values. In Group K, all measurements except the value at the 30th minute were lower than baseline values (Figure 1). Blood pressure (mean arterial pressure-MAP) decreased during the dexmedetomidine infusion, but this effect was not statistically significant (Figure 2). SpO2 values were greater than 95% in all patients. Respiratory rates were kept between 14 and 16.
Figure 1.
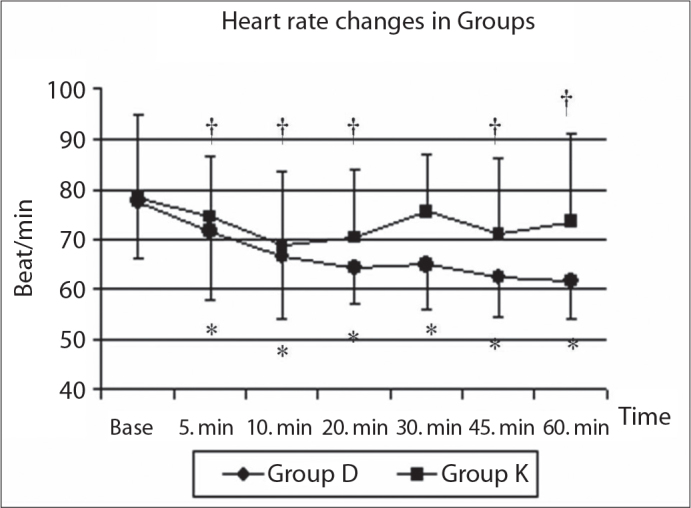
The heart rate changes in both groups.
*statistically significant changes in Group D compared with baseline values; †statistically significant changes in Group K compared with baseline values. The changes of values between groups were not different.
Figure 2.
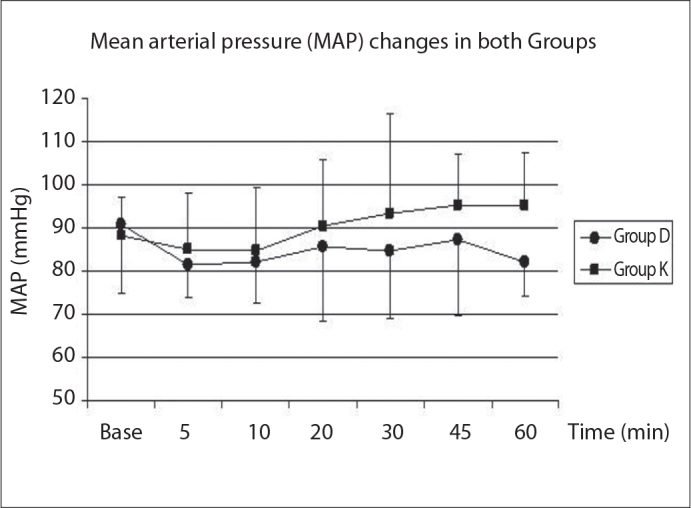
The mean arterial pressure (MAP) changes in all groups.
Mean plasma TAS, TOS and MDA values are shown in Table 3. Patients’ baseline values of MDA, TAS, and TOS were not significantly different between groups (p>0.05). Plasma TOS concentration had a statistically significant decrease at the ATR (after tourniquet release) timepoint (20.09±10.9 μmol H2O2 equiv/L) when compared to baseline (28.47±13.23, μmol H2O2 equiv/L p=0.02) and BTR (before tourniquet release) values (27.37±14.02 μmol H2O2 equiv/L, p=0.001) in Group K. TOS concentrations were slightly decreased at the ATR period compared to the baseline and BTR periods in Group D, but this was not statistically significant.
Table 3.
TAS, TOS and MDA levels in both groups
| Group D (n=15) | Group K (n=15) | p value | |
|---|---|---|---|
| TAS(Base) mmol Trolox equivalent/L | 1.05±0.25 | 1.07±0.34 | NS |
| TAS(BTR) | 0.95±0.28 | 0.88±0.41 | NS |
| TAS(ATR) | 0.96±0.36 | 0.92±0.2 | NS |
| TOS(Base)μmol H2O2 equiv./L | 23.75±12.52 | 28.47±13.23* | NS |
| TOS(BTR) | 20.9±9.2 | 27.37±14.02* | NS |
| TOS(ATR) | 18.79±7.6 | 20.09±10.9 | NS |
| MDA(Base) nmol/mL | 12.57±8.38 | 14.19±8.5 | NS |
| MDA(BTR) | 10.72±7.15 | 15.57±10.58 | NS |
| MD (ATR) | 10.24±5.79 | 1.93±6.35 | NS |
(TAS) total antioxidant status; (TOS) total oxidant status; (MDA) malondialdehyde; (Base) baseline; (BTR) before tourniquet release; (ATR) after tourniquet release; (NS) nonsignificant (p>0.05).
p<0.05 compared with the ATR values in-groups
Complications such as nausea/vomiting, bradycardia/tachycardia, hypotension/hypertension or respiratory depression were not seen.
Discussion
The present study demonstrated that both dexmedetomidine and ketamine infusion attenuated plasma TAS, TOS and MDA production during the early reperfusion period. In the dexmedetomidine group, there were no significant differences between the TAS, TOS and MDA values among the periods. However, in the ketamine group, the TOS values were significantly lower at baseline and in the before tourniquet release period compared with the after tourniquet period. Cardiorespiratory effects were similar between the groups.
The role of anesthetic agents in muscular I/R injury has been evaluated [4, 6, 7]. Dexmedetomidine has been widely investigated in cerebral ischemia models. It has been reported to protect against incomplete ischemia in rats [10] and against focal ischemia in rabbits [14]. A previously conducted study has reported that I/R of the rat kidney results in significant renal injury and that the administration of dexmedetomidine at the starting time of reperfusion can provide varying degrees of protection against renal injury. This study also found better histopathological structure in the kidney of dexmedetomidine treated group rats compared to the untreated group [15].
Yağmurdur et al. [5] examined the effect of dexmedetomidine on I/R injury due to tourniquet application during upper extremity surgery by determining blood MDA and HPX levels. In the dexmedetomidine group, a continuous infusion of dexmedetomidine (1 μg/kg for 10 minutes, followed by 0.5 μg/kg/h) was used until the end of surgery, whereas the control group received an equivalent volume of saline. Venous blood samples were obtained before brachial plexus anesthesia, 1 minute before tourniquet release, and 15 minutes after tourniquet release. They reported that dexmedetomidine significantly attenuated plasma HPX production during ischemia and plasma MDA production in the reperfusion periods compared to those in the control group after reperfusion [5]
We observed similar attenuations in TAS, TOS and MDA levels in both groups, and these changes were not statistically significant between groups. This attenuation may result from the reduction of stress-induced hormones such as adrenaline, noradrenaline and cortisol due to the effects of both ketamine and dexmedetomidine [16, 17].
Our study found no respiratory and hemodynamic differences (MAP and HR) between groups. In-group analysis of the MAP and heart rate found that these values decreased during the procedure, but this reduction did not exceed 20% of the baseline values. Dexmedetomidine has been associated with decreases in HR because of its alpha-2 agonism and sympatholytic effect [18]. In a study, significant decreases in MAP and HR occurred if dexmedetomidine was used as a monotherapy [19]. This may be due to the higher doses of the drug, decreased sympathetic outflow and circulating catecholamine levels [16, 17].
In conclusion, dexmedetomidine provides an adequate sedation and protective effect on I/R injury. This effect is nearly equal to that of ketamine in patients undergoing arthroscopic knee surgery with tourniquet appliance under spinal anesthesia.
Footnotes
Summary of this manuscript is presented in: 41st Turkish Anesthesiology and Reanimation Congress
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Concannon MJ, Kester CG, Welsh CF, Puckett CL. Patterns of free radical production after tourniquet ischemia: Implicationsfor the hand surgeon. Plast Reconstr Surg. 1992;89:846–52. doi: 10.1097/00006534-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Slater TF, Cheeseman KH, Davies MJ, Proudfoot K, Xin W. Free radical mechanism in relation to tissue injury. Proc Nutr Soc. 1987;46:1–12. doi: 10.1079/pns19870003. [DOI] [PubMed] [Google Scholar]
- 3.Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–93. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 4.Saricaoglu F, Dal D, Salman AE, Doral MN, Kilinc K, Aypar U. Ketamine sedation during spinal anesthesia for arthroscopic knee surgery reduced the ischemia-reperfusion injury markers. Anesth Analg. 2005;101:904–9. doi: 10.1213/01.ANE.0000159377.15687.87. [DOI] [PubMed] [Google Scholar]
- 5.Yagmurdur H, Ozcan N, Dokumaci F, Kilinc K, Yilmaz F, Basar H. Dexmedetomidine reduces the ischemia-reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33:941–7. doi: 10.1016/j.jhsa.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Turan R, Yagmurdur H, Kavutcu M, Dikmen B. Propofol and tourniquet induced ischaemia reperfusion injury in lower extremity operations. Eur J Anaesthesiol. 2007;24:185–9. doi: 10.1017/S0265021506001347. [DOI] [PubMed] [Google Scholar]
- 7.Erturk E, Cekic B, Geze S, et al. Comparison of the effect of propofol and N-acetyl cysteine in preventing ischaemiareperfusion injury. Eur J Anaesthesiol. 2009;26:279–84. doi: 10.1097/EJA.0b013e32831c87c7. [DOI] [PubMed] [Google Scholar]
- 8.Maier CM, Sun GH, Kunis DM, Giffard RG, Steinberg GK. Neuroprotection by the N-methyl-D-aspartate receptor antagonist CGP 40116: in vivo and in vitro studies. J Neurochem. 1995;65:652–9. doi: 10.1046/j.1471-4159.1995.65020652.x. [DOI] [PubMed] [Google Scholar]
- 9.Kocoglu H, Karaaslan K, Gonca E, Bozdogan O, Gulcu N. Preconditioning effects of dexmedetomidine on myocardial ischemia/reperfusion injury in rats. Curr Ther Res Clin Exp. 2008;69:150–8. doi: 10.1016/j.curtheres.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–32. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone–alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 14.Maier C, Steinberg GK, Sun GH, Zhi GT, Maze M. Neuroprotection by the alpha 2-adrenoreceptor agonist dexmedetomidine in a focal model of cerebral ischemia. Anesthesiology. 1993;79:306–12. doi: 10.1097/00000542-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kocoglu H, Ozturk H, Ozturk H, Yilmaz F, Gulcu N. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70–4. doi: 10.1080/08860220802546487. [DOI] [PubMed] [Google Scholar]
- 16.Uyar AS, Yagmurdur H, Fidan Y, Topkaya C, Basar H. Dexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull-pin head-holder application during craniotomy. J Neurosurg Anesthesiol. 2008;20:174–9. doi: 10.1097/ANA.0b013e318177e5eb. [DOI] [PubMed] [Google Scholar]
- 17.Aho M, Scheinin M, Lehtinen AM, Erkola O, Vuorinen J, Korttila K. Intramuscularly administered dexmedetomidine attenuates hemodynamic and stres hormone responses to gynecologic laparoscopy. Anesth Analg. 1992;75:932–9. [PubMed] [Google Scholar]
- 18.Kaygusuz K, Gokce G, Gursoy S, Ayan S, Mimaroglu C, Gultekin Y. A comparison of sedation with dexmedetomidine or propofol during shock wave lithotripsy: A randomized controlled trial. Anesth Analg. 2008;106:114–9. doi: 10.1213/01.ane.0000296453.75494.64. [DOI] [PubMed] [Google Scholar]
- 19.De Jonge A, Timmermans PB, Van Zwieten PA. Participation of cardiac presynaptic alpha 2-adrenoceptors in the bradycardic effects of clonidine and analogues. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:8–12. doi: 10.1007/BF00506249. [DOI] [PubMed] [Google Scholar]


