Abstract
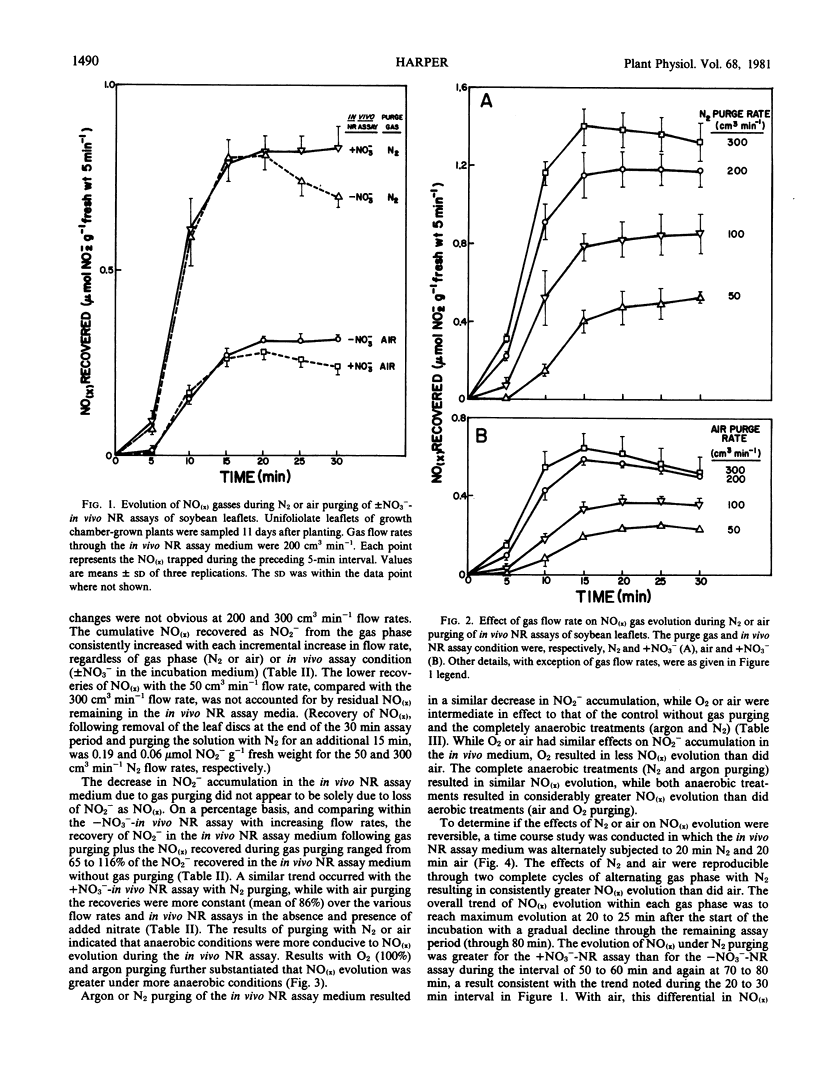
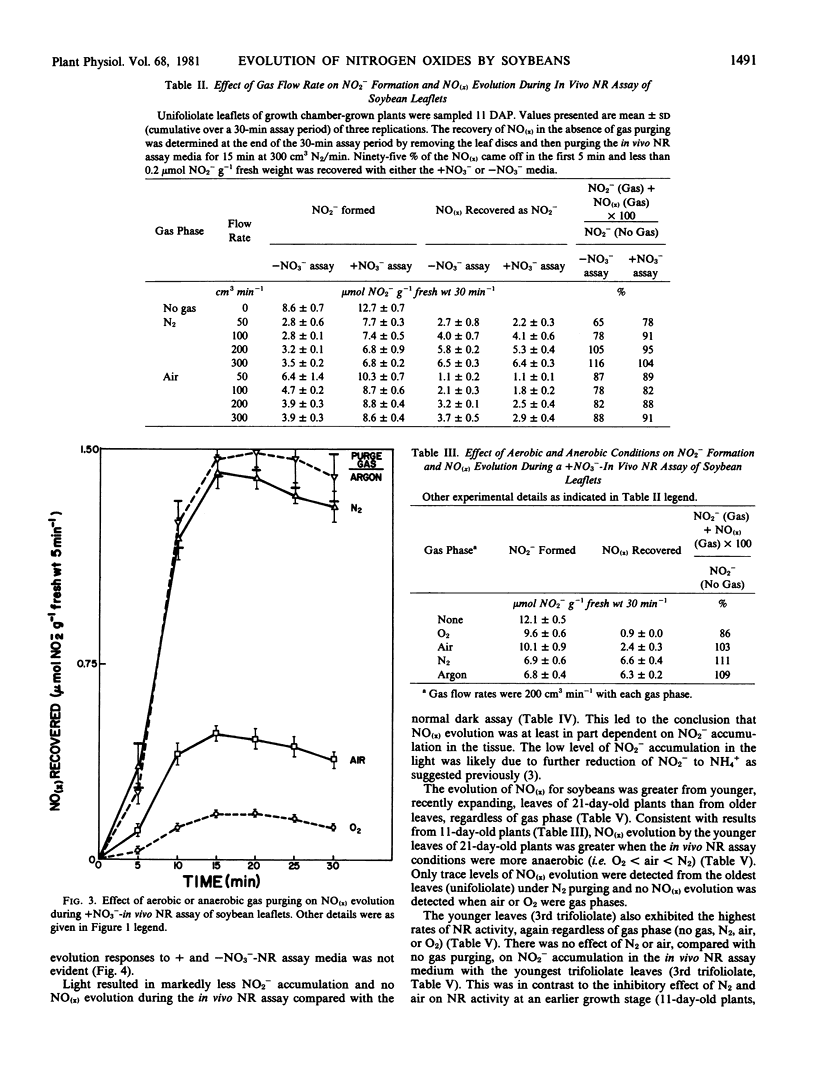
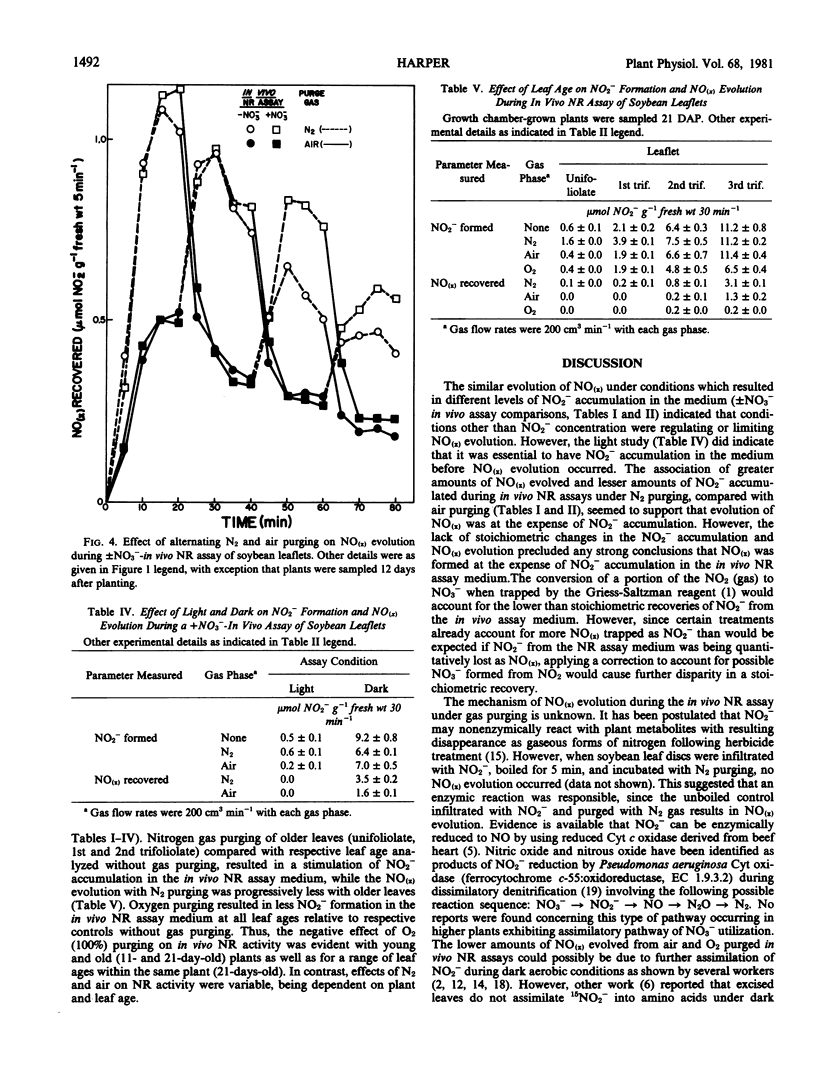
Studies were conducted to quantitate the evolution of nitrogen oxides (NO(x)) from soybean [Glycine max (L.) Merr.] leaves during in vivo nitrate reductase (NR) assays with aerobic and anaerobic gas purging. Anaerobic gas purging (N2 and argon) consistently resulted in greater NO(x) evolution than did aerobic gas purging (air and O2). The evolution of NO(x) was dependent on gas flow rate and on NO2− formation in the assay medium; although a threshold level of NO2− appeared to exist beyond which the rate of NO(x) evolution did not increase further.
The loss of NO(x) from in vivo NR assays under gas purging explains partially, but not stoichiometrically, the decrease in NO2− accumulation in in vivo NR assay medium with young soybean leaves. The lack of stoichiometry between NO(x) evolution and apparent NO2− loss suggests that other mechanisms are also involved in loss of NO2− or inhibition of formation of NO2− during anaerobic and aerobic incubation conditions imposed on the in vivo NR assay of soybean. The mechanism of NO(x) evolution under the assay conditions imposed and the relevance of this phenomenon to intact plants remains unclear.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Hageman R. H. The role of light in nitrate metabolism in higher plants. Photophysiology. 1972;(7):85–113. [PubMed] [Google Scholar]
- Brudvig G. W., Stevens T. H., Chan S. I. Reactions of nitric oxide with cytochrome c oxidase. Biochemistry. 1980 Nov 11;19(23):5275–5285. doi: 10.1021/bi00564a020. [DOI] [PubMed] [Google Scholar]
- Harper J. E. Canopy and Seasonal Profiles of Nitrate Reductase in Soybeans (Glycine max L. Merr.). Plant Physiol. 1972 Feb;49(2):146–154. doi: 10.1104/pp.49.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski E. G. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): I. Effects of Light and Temperature. Plant Physiol. 1976 Dec;58(6):731–735. doi: 10.1104/pp.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton D. C., Weintraub S. T. Identification of nitric oxide and nitrous oxide as products of nitrite reduction by Pseudomonas cytochrome oxidase (nitrate reductase). Biochem Biophys Res Commun. 1980 Nov 17;97(1):236–242. doi: 10.1016/s0006-291x(80)80159-8. [DOI] [PubMed] [Google Scholar]


