Abstract
Objective:
The aim of this study was to determine the antioxidant properties of the L-carnitine (LC) in the treatment of patients with age-related macular degeneration (AMD).
Materials and Methods:
This study involved 60 patients diagnosed with early AMD. The patients were divided into two groups. Group I was the study group that received LC supplementation for 3 months. Group II was the control group and did not consent to LC supplementation over the 3 months. At the end of the 3-month period, markers of lipid peroxidation, malondialdehyde (MDA) and reduced glutathione (GSH) were measured in the two groups.
Results:
In the study group, the MDA level was significantly reduced, while the GSH level was significantly increased at the end of the 3-month period (P<0.001).
Conclusion:
Our results suggest that LC may protect against oxidative damage by decreasing the MDA level, a marker of lipid peroxidation, and increasing GSH.
Keywords: Age-related macular degeneration, Oxidative stress, Malondialdehyde, Glutathione
Özet
Giriş:
Bu çalışmada, yaşa bağlı makular dejenarasyonun (YBMD) tedavisinde antioksidan L-karnitinin (LK) rolünün belirlenmesi amaçlandı.
Gereç ve Yöntem:
Bu çalışma erken YBMD teşhisi konulan 60 hasta üzerinde uygulandı. Hastalar iki gruba ayrıldı. Grup I, çalışma grubu olarak belirlendi ve bu hastalara 3 ay süresince LK tedavisi uygulandı. Grup II, kontrol grubunu oluşturdu ve bu gruptaki hastalara LK tedavisi uygulanmadı. Üç aylık tedavi dönemi sonunda, her iki grupta lipid peroksidasyon göstergesi olan malondialdehit (MDA) glutatyon (GSH) seviyeleri ölçüldü.
Bulgular:
Üç aylık LK tedavisi sonunda, çalışma grubunda MDA düzeyi anlamlı derecede düşerken, GSH düzeyinin anlamlı derecede arttığı belirlendi (P<0,001).
Sonuç:
Bulgularımız, LK tedavisinin, lipid peroksidasyonun bir göstergesi olarak MDA düzeyini düşürerek ve GSH düzeyini arttırarak oksidatif hasara karşı koruma sağladığını gösterdi.
Introduction
Age-related macular degeneration (AMD) is the most common reason of visual loss in individuals over 65 years of age [1,2]. AMD has long been known and widely investigated; however, its etiopathogenesis remains unclear. It is usually seen in advanced ages, and many risk factors have been thought to contribute to the etiology [3,4]. A better understanding of the etiology and pathogenesis of AMD at the cellular and biochemical level is required to improve treatment and prevent this disease. Recent studies have focused on oxidative stress in AMD [5,6]. Some cellular reactions or an insufficiency in the antioxidant defense system have been held responsible for the interruption of the pro-oxidant/antioxidant balance. The central area of the neurosensory retina and the underlying retinal pigment epithelium are likely to be affected by the cumulative toxic effects of continued photic damage. Thus, the free radical hypothesis of aging has been related to AMD, in particular [7–9]. It has been shown that circulating reactive oxygen species (ROS) damage the choriocapillaris and lead to AMD [10]. In the process of aging, there may be oxidative damage in macromolecules such as lipids and proteins because of the disturbed balance between the generation of ROS and ROS clearance. ROS attack double bonds in polyunsaturated fatty acids, inducing lipid peroxidation, which, in turn, results in more oxidative damage [11]. ROS-mediated oxidation of cell membrane lipids yields lipid peroxidation products such as malondialdehyde (MDA) [12]. Increases of serum MDA levels were shown in AMD patients previously [7].
Consequently, cell-protective antioxidant defense mechanisms are activated. In addition, a negative correlation exists between peroxidation and reduced glutathione (GSH). GSH prevents oxidative damage to red blood cells. Considering this property of GSH and the role of MDA in the activation of cell-protective antioxidant defense mechanisms, GSH and plasma MDA concentrations in the blood can be used as markers of oxidative stress [13].
L-carnitine, which is synthesized from lysine and methionine, plays a crucial role in fat metabolism by transporting long-chain fatty acids for the production of energy via β-oxidation and oxidative phosphorylation. It also facilitates the removal of short- and medium-chain fatty acids, which accumulate due to fat metabolism, from the mitochondria [14]. L-carnitine has also been suggested to act in the protection of cell membranes by detoxification of acetyl groups and free coenzyme A [14,15].
Recently, a metabolic therapy, comprised of a combination of omega-3 fatty acids, coenzyme Q10, and acetyl-L-carnitine (ALC), has been introduced for the treatment of early age-related macular degeneration by improving mitochondrial dysfunction. This system specifically improves lipid metabolism and ATP production in the retinal pigment epithelium, improving photoreceptor turnover and reducing the generation of ROS. Thus, according to the findings of Feher et al. [16], metabolic therapy may be the first choice for treating age-related macular degeneration. Furthermore, in another study by Feher et al. [17], the efficacy of a combination of acetyl-L-carnitine, n-3 fatty acids, and coenzyme Q10 on the visual functions and fundus alterations in early AMD was determined. They showed that the visual functions significantly improved in the treatment group by the end of the study period. They also showed a statistically significant decrease in the drusen-covered area of treated eyes compared to eyes treated with a placebo, when either the most affected eyes or the less affected eyes were considered. At the end of the study, the authors concluded that an appropriate combination of compounds that affect mitochondrial lipid metabolism may improve and subsequently stabilize visual functions, and it may also improve fundus alterations in patients affected by early AMD.
Although LC is a well-known antioxidant, there is limited information on its protective effects on lipid peroxidation and the antioxidant system in AMD patients. Thus, in the present study, we tried to determine the effects of LC on the level of lipid peroxidation and the parameters of the antioxidant defense system in patients with AMD.
Materials and Methods
This study is a randomized controlled prospective study that was conducted at the Eye Clinics of Ataturk University. The study involved 60 patients with a diagnosis of early bilateral AMD, a best corrected visual acuity between 0.8 and 0.4 based on the Snellen decimal chart (in the most affected eyes), and within the age range of 55–70 years at the time of enrollment.
Furthermore, the patients who enrolled in the study were not taking any medicinal products for AMD or other clinical conditions. Patients who had ocular diseases such as late AMD (geographic atrophy or macular scarring), exudative retinal disease (including exudative AMD), inherited retinal dystrophies, corneal opacity, cataracts, glaucoma, proliferative vitro retinopathy (PVR), retinal detachment, optic nerve disease, and intraocular inflammatory disease or who had a refractive error greater than +4 D or −6 D were not included. Furthermore, patients who had systemic diseases such as cerebrovascular, hepatic, renal, pulmonary, thyroid or immunosuppressive disorders or who had diabetes were not included. Neither were patients who had a history of alcoholism, smoking, drug abuse, mental disorders, or a history of corticosteroid, phenothiazine, or ant malarial drug use in the month prior to the study or during the study were not included.
The study protocol was approved by the local ethics committee at Ataturk University Medical School, and the study was conducted in accordance with the Helsinki Declaration. All the patients provided their own informed consent before the study.
The detailed ophthalmic and medical history of the patients was obtained, after which all patients were subjected to a complete ophthalmologic examination including slit lamp biomicroscopy of anterior segment, applanation tonometry, funduscopic examination, and fundus fluorescein angiography (FFA). All of the examinations and evaluations were performed by the same physician. Patients with the following characteristics on their FFA were considered early type AMD: the presence of drusen with or without fovea in the macular region and retinal pigment epithelium (RPE) alterations, presenting with hypo- or hyperpigmentation.
No patients have previously received LC treatment. The patients were separated into two groups: the study group (with LC treatment) and the control group (without LC treatment) by randomization. In the light of previous reports, the study group was given 2 capsules containing 100 mg of LC per day to be taken orally for three months. The control group was not given any medicinal products for AMD [16,17]. The composition of medication was determined considering scientific and ethical aspects. The control group was not given any placebo. The patients were provided with detailed information of the efficacy of the drugs used, documented in the literature, and the lack of a classical treatment modality for AMD. Those patients who consented to receiving the drugs were provided with the antioxidant therapy. The patients were asked to refrain from taking any medicinal products for AMD or medications such as corticosteroids, phenothiazine and antimalarial drugs, during the month before the study and during the 3-month study period. No restrictions were applied to the use of drugs by the patients for clinical conditions other than the pathologies determined during this study. However, patients were asked to report any dose adjustments or new therapies for all pathological events that occurred during the study.
All 60 patients were examined during follow-up and analyzed throughout the study. Serum MDA and GSH levels were measured in the study and control group at the baseline and at the end of 3-month treatment period.
Malondialdehyde and Glutathione Assays
Blood was collected by venous arm puncture in both groups. The collected blood was injected into EDTA vaccutainers, and the plasma was separated by centrifuging at 1.000 g for 15 minutes. MDA concentrations in plasma were measured by high performance liquid chromatography with fluorescent detection (HPLCFLD), as previously described [18]. Briefly, 50 µL plasma was mixed in 0.44 M H3PO4 and 42 mM thiobarbituric acid (TBA), and incubated for 30 min in a boiling water bath. After rapidly cooling on ice, an equal volume of alkaline methanol was added to the sample, then the sample was vortexed and centrifuged (3000 rpm for 3 min) and the aqueous layer was removed. Next, a 20 µL aliquot of supernatant was analyzed by HPLC (HP, Agilent 1100 modular systems with FLD detector, Germany): Column, RP-C18 (5 µm, 4.6 × 150 mm, Eclipse VDB- C18. Agilent); elution, methanol (40:60, v/v) containing 50 mM KH2PO4 buffer (pH 6.8); flow rate, 0.8 mL/min. Fluorometric detection was performed with excitation at 527 nm and emission at 551 nm. The peak of the MDA-TBA adduct was calibrated with a 1,1,3,3-tetraethoxypropane standard solution treated in exactly the same manner as the plasma and urine samples. The level of GSH was determined according to the Ellman method [19]. The total GSH level was measured spectrophotometrically at 412 nm and the results were expressed as nmol/L.
Data for biochemical analyses are expressed as mean and standard deviation (SD). Statistical analyses were performed using SPSS for Windows (version 11.0, SPSS Inc., Chicago, IL, USA). Paired samples t test was used to determine statistical significance. P<0.05 was considered statistically significant.
Results
The mean age of participants was 59.16 ± 12.21 years (range: 53–72 years) in the study group and 58.76 ± 10.60 (range: 52–74 years) in control group. Seventeen subjects of the study group and 19 of the control group were male. There were no differences in the age and gender between patients in the treated and control groups (P>0.05).
The baseline (before LC therapy) plasma MDA level of the study group was 6.857 ± 1.02 nmol/ml and the plasma GSH was 6.687 ± 1.08 nmol/L, while the baseline plasma MDA level of the control group was 6.741 ± 1.00 nmol/ml and the plasma GSH was 6.567 ± 1.75 nmol/L (Table 1). At the end of 3 months, the plasma MDA level of the study group was 4.323 ± 1.24 nmol/ml and the plasma GSH was 8.517 ± 1.1 nmol/L (Table 1). However, in the control group, the mean plasma MDA level was 7.099 ± 1.34 nmol/ml and the plasma GSH was 6.48 ± 1.25 nmol/L (Table 1). The plasma MDA and GSH levels of the study group were significantly different from the baseline levels of the study group (P<0.001). No significant differences were observed in the control group for plasma MDA and GSH levels (P>0.05) (Figures 1, 2).
Table 1.
The mean change of malondialdehyde (MDA) and glutathione (GSH) in patient and control groups at pre- and past-L-camitine treatment.
| Parameters | Study group (n=30) | Control group (n=30) |
|---|---|---|
| MDA level (nmol/ml) (before therapy) | 6.857±1.02a | 6.741±1.00 |
| MDA level (nmol/ml) (after therapy) | 4.323±1.24b,c | 7.099±1.34 |
| GSH level (nmol/L) (before therapy) | 6.687±1.08a | 6.567±1.75 |
| GSH level (nmol/L) (after therapy) | 8.517±1.1b,c | 6.48±1.25 |
Values are expressed as means ± SD,
Study group vs. control group (P>0.05),
After therapy vs. before therapy (P<0.001),
Study group vs. control group (P<0.001)
Fig. 1.
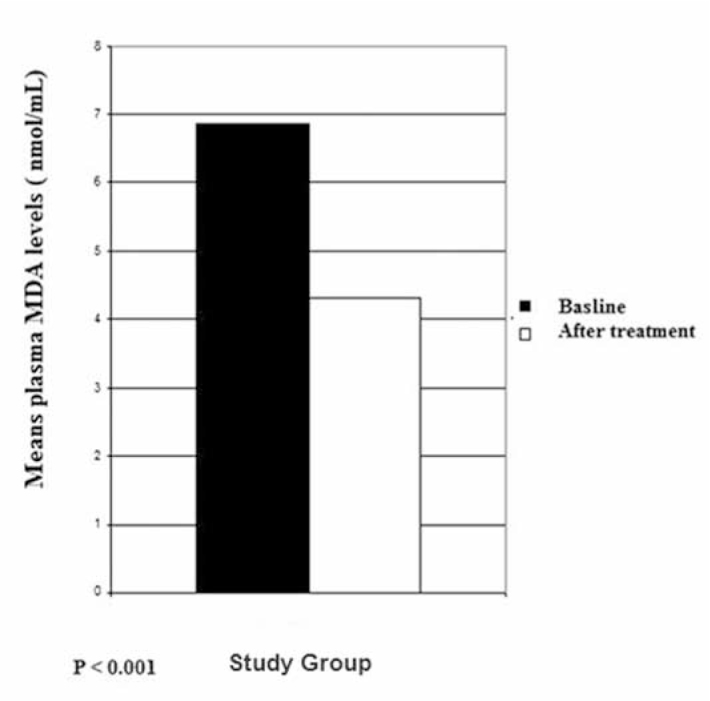
Mean plasma malondialdehyde (MDA) levels in patients.
Fig. 2.
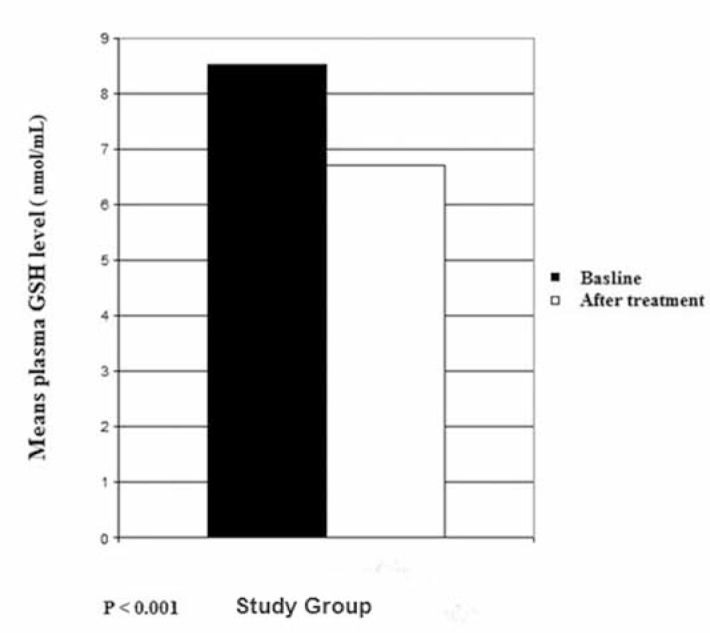
Mean plasma glutathione (GSH) levels in patients.
Three patients in the study group had diarrhea one week after the treatment. These patients were consulted at the gastroenterology department, and their treatment doses were halved for one week. Upon resolution of these symptoms after one week, the initial dose was resumed. These patients did not show further significant adverse effects during the study period. All patients, including the three with diarrhea, were included in the analysis at end of the study.
Discussion
Because ROS contain an unpaired electron, they are highly reactive. All tissues produce ROS during aerobic metabolism, and they can also be formed by photochemical reactions [11]. ROS lead to cell damage and lipid peroxidation by impairing the protective systems. All of these ROS mechanisms are important because they may underlie the pathogenesis of a number of acute and chronic diseases, as well as the pathogenesis of AMD [7,10]. Because the eye is continuously exposed to light throughout life, the risk of damage by oxidative mechanisms is high. The biochemical composition of posterior segment structures (unsaturated fatty acids) is thought to make eye more susceptible to damage than other organs. The retina has high polyunsaturated fatty acid content of photoreceptor membranes; thus, it is at increased risk of lipid peroxidation by ROS [20,21]. ROS attack double bonds in polyunsaturated fatty acids, and thus induce lipid peroxidation, which then results in more oxidative damage [20–22]. ROS mediated oxidation of cell membrane lipids are responsible for the formation of lipid peroxidation products, such as MDA [11]. In our study, the AMD patients had higher MDA levels than the controls, which is compatible with the findings of some earlier studies [20,21].
Oxidative stress, likely to develop due to an enormous production of ROS, has been held responsible for increases in lipid peroxidation, changes in GSH levels, and related enzymatic activities. Thus, the presence of a functional defense mechanism against oxidative stress mainly related to the glutathione metabolism seems likely [23,24]. Similarly, in our study, AMD patients with higher plasma MDA levels showed lower plasma GSH levels. In earlier reports, LC has been shown to play a role in preventing lipid peroxidation and supporting the antioxidant system against oxidative damage in the experimental model, which is consistent with the findings of our study [23–27]. Some authors have reported that LC is a cofactor in the transfer of long-chain fatty acids, allowing the β-oxidation of fatty acids in the mitochondria; LC is also a known antioxidant with protective effects against lipid peroxidation. Some authors concluded that administration of LC significantly reduced acetaminophen-induced elevations in AST, ALT, total sialic acid, and MDA concentrations, as well as increased GSH levels at all sampling points [28]. In a study by Citil et al., the cell-protective effect of ALC could be used to inhibit the genotoxic, mutagenic, and cell proliferative effects of MDA. The authors also concluded that one of the important toxic aldehydes of lipid peroxidation, as well as the increase of GSH concentration, might be due to the decreased lipid peroxidation caused by LC supplementation [29].
In a more recent study, Shamsi et al. [30] studied the efficacy of LC against oxidative changes in human retinal pigment epithelium (RPE) cells. In their study, the authors suggest that the change in the antioxidant potential of the RPE induced by oxidative stress was restored by LC treatment; this restoration was demonstrated by an increase in GSH and SOD activities. Thus, the authors speculated that LC is capable of protecting RPE cells from H2O2-induced oxidative damage, implying that micronutrients can have a positive effect and can play an important role in the treatment of oxidation-induced ocular disorders.
In conclusion, this study demonstrates that LC treatment enhanced the plasma GSH level in AMD patients. This antioxidant effect may be due to the decrease of lipid peroxidation caused by L-carnitine treatment in AMD patients. While our results have provided preliminary data on the clinical significance of treating early AMD, further studies with larger sample sizes are required to support this hypothesis, and above all, to elucidate the pathophysiology of AMD.
Footnotes
Conflict interest statement The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Neely KA, Bressler NM, Bressler SB. Clinical characteristics, epidemiology and natural history of age related macular degeneration. Ophthalmol Clin North Am. 1996;6:291–306. [Google Scholar]
- 2.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: Follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–26. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 3.Harris A, Chung HS, Ciulla TA, Kagemann L. Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog Retin Eye Res. 1999;18:669–87. doi: 10.1016/s1350-9462(98)00037-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;4:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Nelson KC, Wu M, Sternberg PJ, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 6.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;7:1280–7. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surg Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Oxygen is a toxic gas-an introduction to oxygen toxicity, and reactive oxygen species. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 1999. pp. 7–17. [Google Scholar]
- 9.Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–9. [PubMed] [Google Scholar]
- 10.Marshall J. The ageing retina: physiology or pathology. Eye. 1987;1:282–95. doi: 10.1038/eye.1987.47. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Reactive oxygen species in living system: source, biochemistry, and role in human disease. Am J Med. 1991;91:14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 12.Shrilatha B, Muralidhara Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int J Androl. 2007;30:508–18. doi: 10.1111/j.1365-2605.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaidi SM, Al-Qirim TM, Banu N. Effects of anti-oxidant vitamins on glutathione depletion and lipid peroxidation induced by restraint stress in the rat liver. Drugs R D. 2005;6:157–65. doi: 10.2165/00126839-200506030-00004. [DOI] [PubMed] [Google Scholar]
- 14.Bertelli A, Conte A, Ronca G. L-propionyl carnitine protects erythrocytes and low density lipoproteins against peroxidation. Drugs Exp Clin Res. 1994;20:191–7. [PubMed] [Google Scholar]
- 15.Di Giacomo C, Latteri F, Fichera C, et al. Effect of acetyl-L-carnitine on lipid peroxidation and xanthine oxidase activity in rat skeletal muscle. Neurochem Res. 1993;18:1157–62. doi: 10.1007/BF00978367. [DOI] [PubMed] [Google Scholar]
- 16.Feher J, Kovacs B, Kovacs I, Schvoller M, Corrado Balacco G. Metabolic therapy for early treatment of age-related macular degeneration. Orv Hetil. 2007;48:2259–68. doi: 10.1556/OH.2007.28250. [DOI] [PubMed] [Google Scholar]
- 17.Feher J, Kovacs B, Kovacs I, Schveoller M, Papale A, Balacco-Gabrieli C. Improvement of visual functions and fundus alterations in early age-related macular degeneration treated with a combination of acetyl-L-carnitine, n–3 fatty acids, and coenzyme Q10. Ophthalmologica. 2005;219:154–66. doi: 10.1159/000085248. [DOI] [PubMed] [Google Scholar]
- 18.Li K, Shang X, Chen Y. High-performance liquid chromatographic detection of lipid peroxidation in human seminal plasma and its application to male infertility. Clin Chim Acta. 2004;346:199–203. doi: 10.1016/j.cccn.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaemmerer E, Schutt F, Krohne TU, Holz FG, Kopitz J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest Ophthalmol Vis Sci. 2007;3:1342–7. doi: 10.1167/iovs.06-0549. [DOI] [PubMed] [Google Scholar]
- 21.Ohira A, Ueda T, Ohishi K, Hiramitsu T, Akeo K, Obara Y. Oxidative stress in ocular disease. Nippon Ganka Gakkai Zasshi. 2008;112:22–9. [PubMed] [Google Scholar]
- 22.Nowak M, Gnitecki W, Jurowski P. The role of retinal oxygen metabolism in origin of age-related macular degeneration (AMD) Klin Oczna. 2005;107:715–8. [PubMed] [Google Scholar]
- 23.Stocker R. Induction of heme oxygenase as a defense against oxidative stress. Free Radic Res Commun. 1990;9:101–12. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 24.Scibior D, Skrzycki M, Podsiad M, Czeczot H. Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin Biochem. 2008;41:852–8. doi: 10.1016/j.clinbiochem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Abraham NG, Lin JHC, Schwartzman ML, Levere RD, Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1988;20:543–58. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- 26.Jaswal S, Mehta HC, Sood AK, Kaur J. Anti-oxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2003;338:123–9. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tastekin N, Aydogdu N, Dokmeci D, et al. Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res. 2007;56:303–10. doi: 10.1016/j.phrs.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Yapar K, Kart A, Karapehlivan M, et al. Hepatoprotective effect of L-carnitine against acute acetaminophen toxicity in mice. Exp Toxicol Pathol. 2007;2:121–8. doi: 10.1016/j.etp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Citil M, Gunes V, Atakisi O, Ozcan A, Tuzcu M, Dogan A. Protective effect of L-carnitine against oxidative damage caused by experimental chronic aflatoxicosis in Quail (Coturnix Coturnix) Acta Veterinaria Hungarica. 2005;3:319–24. doi: 10.1556/AVet.53.2005.3.5. [DOI] [PubMed] [Google Scholar]
- 30.Shamsi FA, Chaudhry IA, Boulton ME, Al-Rajhi AA. L-carnitine protects human retinal pigment epithelial cells from oxidative damage. Curr Eye Res. 2007;32:575–84. doi: 10.1080/02713680701363833. [DOI] [PubMed] [Google Scholar]


