Abstract
Objective:
COX-2-selective inhibitors are used in the prevention and management of colorectal carcinogenesis. Our objective was to investigate if COX-2 levels have prognostic value in patients diagnosed with colorectal carcinoma.
Materials and Methods:
This is a retrospective study of 112 patients diagnosed with colorectal carcinoma between 2000–2004 from the General Surgery Department at Haydarpasa Training Hospital, Gulhane Military Medical Academy. Patients were assessed according to age, gender, localization of the tumor, stage of the tumor, remote metastasis status, patient survival, COX-2 levels and grade of differentiation.
Results:
COX-2 levels significantly affect the duration of survival (P=0.026) and overall survival (P=0.013). The COX-2 significance value showed a tendency to change from negative to positive while a statistically meaningful decrease was observed in the survival value (r=−0.25; P=0.007) in groups related with the survival duration of cases (r=−0.24; P=0.01). The median survival was 36 (26.35–45.65) months. During the examination of survival statuses of cases, a statistically meaningful difference was determined between patients whom were alive and dead (P=0.01).
Conclusion:
We conclude that COX-2 levels are a negative predictor of survival.
Keywords: Cyclooxygenase-2, Rectal carcinoma, Prognosis
Özet
Giriş:
COX-2 selektif inhibitörü plan ajanlar kolorektal kanserlerde koruyucu olarak ve tedavide kullanılmaktadır. Çalışmamızda amaç COX-2 değerlerinin kolorektal kanser tanısı almış hastalardaki prognostik önemini değerlendirmektir.
Gereç ve Yöntem:
Çalışma 2000–2004 yılları arasında GATA Haydarpaşa Eğitim hastanesi Genel Cerrahi Servisi’nde kolorektal kanser tanısı almış 112 hastanın retrospektif değerlendirilmesiyle yapıldı. Hastalar yaş, cinsiyet, tümör lokalizasyonu, evre, yaşam süresi, COX-2 seviyesi ve differensiasyon derecesine göre değerlendirildi.
Bulgular:
COX-2 değerinin negatifden pozitife doğru değişmesi, beklenen yaşam süresinde istatistiksel olarak anlamlı azalmayla (r=−0,25; P=0,007) ve yaşam süresinde de düşüşle (r=−0,24; P=0,01) ilgili bulunmuştur. Olgulardaki median yaşam süresi 36 (26,35–45,65) ay olarak bulundu. Beş yıllık süre sonunda COX-2 pozitif olanlarda yaşam şansı istatistiksel olarak anlamlı derecede vfarklıydı (P=0,01).
Sonuç:
COX-2 pozitifliği beklenen yaşam süresini olumsuz etkileyen kötü prognostik bir faktördür.
Introduction
One signaling pathway that affects tumor development is mediated by the cyclooxygenase (COX) enzyme. COX signals via Prostaglandin E2 and Prostaglandin I2, and thereby influences carcinogenesis. Comparisons of colon mucosa from malignancies with normal colon tissue show that COX-2 expression is often elevated in tumors, whereas COX-1 expression is usually unchanged [1–4].
Cancer cells lack or have inactivated the internal mechanisms that lead to apoptosis [5]. Previous reports suggest that COX-2 inhibits cellular apoptosis [6], regulates angiogenesis [7], and accelerates the growth, angiogenesis and metastatic potential of a tumor [8]. Accordingly, groups have concluded that COX-2 elevation contributes to carcinogenesis [9–11]. As a result, selective COX-2 inhibitors are often studied for the prevention and management of colorectal tumors [1,3,4,12–14].
Matrix metalloproteins (MMP) are responsible for the degranulation of the matrix. This may impair the basal membrane of the tissue where a tumor is located, leading to deformation of the extra cellular matrix and subsequent progression of the tumor. Inhibition of prostaglandins may halt the inhibition of MMP, which may decrease tumor invasiveness [15].
The Coxib group of drugs are selective COX-II inhibitors. They can inhibit the growth of colon tumors by decreasing angiogenesis, increasing apoptosis, and reducing cellular proliferation [8]. Celecoxibin shows dose-dependent inhibition of tumor growth in rats [16]. In another study of 66,500 adults who received NSAID drugs, mortality due to gastrointestinal and colon cancers was decreased by 50% [17]. COX-2 inhibition causes arachiodonic acid to accumulate in cells, leading to an increase in ceramide. Ceramide is a potent inducer of apoptosis [1].
Tumors that have high COX-2 expression levels show a high degree of vascular invasion, whereas COX-2 inhibition leads to an 80% decrease in angiogenesis [1]. The mechanisms underlying these angiogenic effects are unclear.
The objective of our study was to investigate COX-2 as a prognostic indicator in our series of colorectal cancer patients with respect to age, gender, stage, degree of differentiation, survival period and the presence of distant metastases.
Materials and Methods
We undertook a retrospective analysis of 112 patients treated surgically for colorectal cancer (CRC) between 2000–2004. Patients who did not receive neoadjuvant therapy were classified according to age, gender, localization of the tumor, stage of disease, differentiation, presence of metastases, survival and COX-2 expression.
Preparation of the Drug Product
Two cross-sections 4 microns thick were prepared from paraffin-embedded tumor blocks. One of the cross-sections was stained with hematoxylin and eosin, and then the tumor was classified according to histopathological type. TNM staging was performed using information from the patients’ medical record and pathology reports.
Immuno-Histochemical Assessment
To quantify COX-2 expression, other cross-sections were prepared by a Tissue Macro Array method and immunostained using a COX-2 anti-human monoclonal antibody kit (sp21) (LABVISION).
Scoring Method
At the end of immuno-histochemical stain (IHCS) process, immunoreactive tumor cells contained a stained cytoplasm. Scoring was based on staining intensity: 1 indicated low intensity, 2 was moderate intensity, and 3 indicated strong staining. Tumors that scored a 3 were considered COX-2-positive, and those that scored a 1 or 2 were considered negative.
Statistical Analyses
Statistical analyses were performed using SPSS (Statistical Package for Social Sciences), for Windows 10.0 program. The Kaplan-Meier method was used to carry out survival analyses and a log rank test was applied to determine the predictive value of COX-2 expression. The Q-square test was applied to determine the impact of COX-2 significance on gender, survival time, localization of the tumor and differentiation. A p<0.05 value was used as the threshold for statistical significance.
Results
Patient characteristics and demographic data are summarized in Table 1.
Table 1.
Characteristics of the tumor and demographic data
| Demographic features | n (%) | COX-2 negative | COX-2 positive | P | ||
|---|---|---|---|---|---|---|
| Age | 60 years old and over | 87 (77.6%) | 12 | 75 | ||
| Below 60 years | 25 (22.4%) | 4 | 21 | 0.75 | ||
| Gender | Female | 46 (41.07%) | 8 | 38 | ||
| Male | 66 (58.93%) | 8 | 58 | 0.43 | ||
| Tumor localization | Caecum | 7 (6.25%) | 11 | 54 | ||
| Ascending colon | 17 (%15.18) | |||||
| Transverse colon | 4 (3.57%) | |||||
| Descending colon | 15 (13.39%) | |||||
| Sigmoid colon | 22 (19.64%) | |||||
| Rectum | 47 (41.96%) | 5 | 42 | 0.34 | ||
| Tumor type | Adenocarcinoma | 108 (96.43%) | ||||
| Signet ring | 2 (1.79%) | |||||
| Mucinous | 1 (0.89%) | |||||
| Melanoma | 1 (0.89%) | |||||
| Tumor stage | 0 | 0 (0.0%) | 0 | 0 | ||
| 1 | 26 (23.21%) | 5 | 21 | |||
| 2 | 41 (36.61%) | 7 | 34 | |||
| 3 | 33 (29.46%) | 4 | 29 | |||
| 4 | 12 (10.71%) | 0 | 12 | 0.41 | ||
| Tumor differentiation | Good | 23 (20.54%) | 3 | 20 | ||
| Moderate | 74 (66.07%) | 13 | 76 | |||
| Poor | 15 (13 39%) | 0.99 | ||||
| Tumor metastasis | No | 102 (91.07) | 16 | 86 | ||
| Yes | 17 (8.93) | 0 | 10 | 0.54 | ||
| Survival | 0–2 years | 39 (34.84%) | 2 | 37 | ||
| 2–5 years | 17 (15.18%) | 1 | 16 | |||
| Still alive | 56 (50.005) | 13 | 43 | 0.026 | ||
Out of 112 patients enrolled in the study, 96 (86%) tumors stained positively for COX-2. We did not observe a significant relationship between patient gender and COX-2 overexpression (P=0.43). Eight (17%) of 46 tumors from female patients were negative for COX-2, whereas 38 (83%) tumors stained positively. Of the 66 male patients, 58 (88%) were positive for COX-2 and eight (12%) were negative.
The mean age of patients was 66.5 years (range 21–89). Eighty-seven (78%) patients were at least sixty years old, where as 25 (22%) were under the age of 60. Of the 87 patients sixty and older, 75 (86%) had tumors that were positive for COX-2, as compared with 21 (84%) positive tumors out of the 25 patients under 60 years old. We did not observe a significant relationship between COX-2 expression and age (P=0.75).
When we analyzed COX-2 expression with respect to localization of the tumor, we found that 54 (83%) tumors tested positive of the 65 resected from the colon, and 42 (89%) of the 47 rectal tumors were immunoreactive for COX-2. Thus, there is no significant relationship between the localization of the tumor and COX-2 positivity (P=0.34).
Next, we analyzed the differentiation of each tumor. Twenty (87%) of 23 well differentiated tumors expressed COX-2, as compared with 89 moderately differentiated and 76 (85%) poorly differentiated tumors. No significant relationship was found between tumor differentiation and COX-2 expression (P=0.99).
We evaluated the primary tumors of patients with known metastases. All ten patients who had distant metastases had tumors that expressed COX-2, and 86 of the 102 patients without remote metastases had tumors positive for COX-2 expression. No any statistically significant difference was found between the presence of remote metastases and COX-2 expression (P=0.54).
COX-2 was expressed in 21 of 26 patients with Stage I disease, 41 of 48 patients in Stage II, and 29 of 33 patients in Stage III. Tumors from all 12 of the Stage IV patients enrolled in this study tested positive for COX-2. Based on these results, we found no significant relationship between tumor stage and COX-2 expression (P=0.41).
We grouped patients into three categories according to survival time from diagnosis: 0–2 years, 2–5 years, and still alive (Table 2). We found that decreased COX-2 expression is correlated with increased survival (P=0.026).
The median survival time of patients in this study was 36 months [range 26–46]. The median survival time of patients with COX-2-positive tumors was 33 (+4,55) months, whereas the median survival for patients with COX-2-negative tumors was 58 (+4,31) months. Thus, our study revealed a significant difference in survival time between patients with tumors that express COX-2 versus those that do not (p=0.01). The five-year survival was under 40% for patients with COX-2-positive tumors. In contrast, patients with COX-2-negative tumors demonstrated an 80% five year survival rate.
Discussion
Thun et al [17] demonstrated that COX-2 is absent in the normal colonic mucosa while the majority (73%) of patients with colorectal cancers express this protein in this tissue. Similarly, Takeyoshi et al [18] reported that COX-2 is absent in the normal mucosa but is present in 71.6% of the patients with colorectal cancer. Of the 112 cases of colorectal tumors included in our study, 86% expressed COX-2. Fujita et al [19] found that tumor invasiveness increases with increased COX-2 expression.
Previous studies have not found a correlation between COX-2 expression and the age or gender distribution of patients [9,11,18,20–22]. Our study confirms this result.
Discordant accounts of the relationship between COX-2 expression and colorectal tumor localization are available in the literature. Two separate studies by Thun et al [17] and Gialdiello et al [20] report that rectal tumors exhibit less COX-2 expression. An alternative study by Dimburg et al [23] contradicts these results. Several ongoing studies have failed to demonstrate a significant relationship between COX-2 expression and the localization of the tumor [9,16,18,22,24]. Our study revealed no correlation between COX-2 expression and tumor localization.
Though we did not observe a significant relationship between tumor differentiation and COX-2 expression, all of our cases of poorly differentiated tumors expressed COX-2. A larger-scale study is likely to reveal a correlation between poorly differentiated tumors and high levels of COX-2 expression.
Since we only had 12 patients with Stage IV disease, and all of these tumors were either moderately or poorly differentiated, we could be missing significant effects of COX-2 on stage or metastatic properties of these tumors.
Dubois et al [2] and Tsuji et al [7] found a correlation between increased COX-2 expression and advanced stage tumors. Xiong et al [22] also reported an increase in COX-2 expression in Stage III and IV tumors. In our series, we failed to find a significant relationship between the tumor stage and COX-2 expression. However, all cases of Stage IV disease stained positive for COX-2.
In their studies, Yamauchi et al [8] and Tsuji et al [7] failed to demonstrate a statistically significant correlation between COX-2 and the presence of metastases, but they found COX-2 expression in all cases with metastases. We had a similar result in all ten of our metastatic cases, but this did not achieve statistical significance. Furthermore, comparison of results with the mean COX-2 expression which was demonstrated in the literature regarding patients with a colorectal cancer, due to a 86% ratio of positivism, our ratio was slightly higher than the ratio indicated in the literature, as it designated an increase in the expression of COX-2, but was not accepted as a clear prognostic factor.
In our series, COX-2 expression correlated with a significant decrease in survival time. In COX-2-positive patients, the median survival time was 33 months, as compared with 58 months for COX-2-negative patients. This confirms the results of Masunaga et al [25], who found that the five year survival of COX-2-positive patients was 40.5%, compared with 91.6% for COX-2-negative patients. Our study of 112 patients shows that COX-2 positivity is correlated with decreased two-year disease-free survival and five-year overall survival. Therefore, we conclude that COX-2 expression may be a negative prognostic indicator. Since the majority of the patients (80%) had moderately and poorly differentiated tumors and our series contained a small number of early stage patients, our study may underestimate the effect of COX-2 expression on prognosis.
To date, COX-2 expression is not considered to be an effective prognostic factor for tumor aggressiveness or to direct clinical management. We propose that large-scale prospective studies should be carried out to investigate the impact of COX-2 expression on survival time. Additionally, though they did not achieve statistical significance in our study, the relationships between COX-2 expression and decreased differentiation, increased metastasis, and advanced stage suggests that COX-2 may be an indicator of poor prognosis. Accordingly, we suggest that COX-2 expression negatively affects disease free and overall survival times.
Fig. 1.
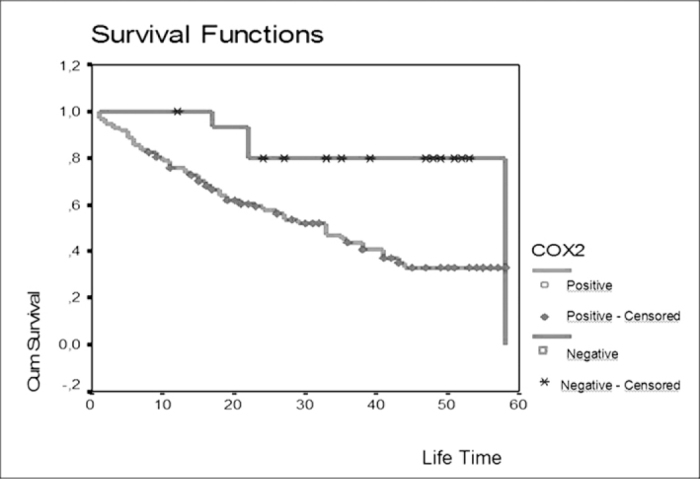
Patient survival
Footnotes
Conflict interest statement The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Charch RD, Fleshman JD, McLeod HL. Cyclooxygenase 2 inhibition in colorectal cancer therapy. Br J Surg. 2003;90:1055–67. doi: 10.1002/bjs.4297. [DOI] [PubMed] [Google Scholar]
- 2.Dubois RN, Tsuji M, Bishop P, Awad JA, Makita K, Lanahan A. Cloning and characterization of a growth factor-induble cyclooxygenase gene from rat intestinal epithelial cells. Am J Physiol. 1994;266:822–7. doi: 10.1152/ajpgi.1994.266.5.G822. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Egan KM, Hunter DJ. Aspirin and the risk of colorectal cancer in woman. N Engl J Med. 1995;333:609–14. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 4.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–8. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volgelstein B, Faeron ER, Hamilton SR. Genetic alterations during colorectal tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji M, Dubois RN. Alterations in cellular adhesion and apopitosis in epithelial cells over-expressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji M, Kawano S, duBoris RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamauchi T, Watanabe M, Kubota T, Hasegawa H, Ishii Y, Endo T. Cyclooxygenase-2 expression as a new marker for patients with colorectal cancer. Dis Col Rec. 2002;45:98–103. doi: 10.1007/s10350-004-6120-5. [DOI] [PubMed] [Google Scholar]
- 9.Eberhart CE. Up-regulation of COX-2 gene expression in human coclorectal adenomas and adenocarsinomas. Gastroenterology. 1994;107:1183. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 10.Ottino P, Bazen HE. Corneal stimulation of MMP-1,9 and uPA by platelet-activating factor is mediated by COX-2 metabolites. Curr Eye Res. 2001;23:77–85. doi: 10.1076/ceyr.23.2.77.5471. [DOI] [PubMed] [Google Scholar]
- 11.Sano H. Expression of COX-1 and COX-2 in human colorectal cancer. Cancer Res. 1995;55:3785–9. [PubMed] [Google Scholar]
- 12.DuBois RN, Abramson SB, Crafford L. COX in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 13.Kishimato Y. Effects of long term administration of sulindav on APC mRNA and apopitosis in colons of rats treated with azoxymethane. J Cancer Res Clin Oncol. 2002;128:589–95. doi: 10.1007/s00432-002-0384-8. [DOI] [PubMed] [Google Scholar]
- 14.Kishimato Y. Effects of COX-2 inhibitor NS-398 on APC and c-myc expression in rat colon carcinogenesis induced by azoxymethane. J Gastroenterol. 2002;37:186–93. doi: 10.1007/s005350200019. [DOI] [PubMed] [Google Scholar]
- 15.Nzelson AR, Fingleton B, Rothenberg ML. Matrix metalloproteinases biologic activity and clinical imolications. J Clin Oncol. 2000;18:1135–49. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 16.Masferrer JL. Antiangiogenic and antitumor activites of COX-2 inhibitors, Cancer Re. 2000;60:1306–11. [PubMed] [Google Scholar]
- 17.Thun MJ, Nambodiri MM, Hearth CW. Aspirin use and reduced risk of fatal colon cancer. New Engl J Med. 1991;325:1593–96. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 18.Takeyoshi Y, Masahiko W, Tetsuro K. COX-2 expression as a new marker for patients with colorectal cancer. Dis Col Rectum. 2001;45:98–103. doi: 10.1007/s10350-004-6120-5. [DOI] [PubMed] [Google Scholar]
- 19.Fujita T, McLeod HL. Tumor markers of prognosis in colorectal cancer. Br J Cancer. 1999;79:191–203. doi: 10.1038/sj.bjc.6690033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gialdiello FM, Hamilton SR, Krush AJ. treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 21.Isakson P. Discovery of a better inductable COX-2 in vivo is inflammatory. Proc Natl Acad Sci USA. 1994;91:3228–32. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong B, Sun TJ, Hu WD. Expression of cyclooxygenase-2 in colorectal cancer and its clinical significance. W J Gastroenterol. 2005:1105–8. doi: 10.3748/wjg.v11.i8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimburg J, Samuelsonn A, Hugander A. Differantial expression of COX-2 in human colorectal cancer. Gut. 1999;45:730–2. doi: 10.1136/gut.45.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart M, Macrae FA, Williams CB. Neoplasia and ureterosigmoidostomy: A colonoscopy survey. Br J Surg. 1982;69:414–7. doi: 10.1002/bjs.1800690720. [DOI] [PubMed] [Google Scholar]
- 25.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–8. [PubMed] [Google Scholar]


