Abstract
Objective:
The aim of this study is to compare the effect of transrectal power Doppler ultrasound (PDUS) and gray scale transrectal ultrasound (TRUS) for the diagnosis of prostate cancer.
Materials and Methods:
Seventy-six patients evaluated with transrectal PDUS and TRUS underwent eight systematic TRUS guided core-needle biopsies, with additional cores from abnormal areas. Histologic diagnoses were classified as benign prostatic hyperplasia, chronic prostatitis, intraepithelial neoplasia and adenocarcinoma. TRUS and PDUS findings of the cases were recorded.
Results:
PDUS sensitivity, specificity, positive predictive value (PPV) and negative predictive values were 81%, 81%, 54% and 94%, respectively. PDUS had a greater sensitivity and specificity than TRUS (43% and 60%, respectively) and identified cancer cases more accurately (Table 2).
Conclusion:
Hypervascular foci in PDUS signify suitable zones for biopsy. When combined with systematic TRUS guided biopsy, PDUS increases the cancer detection rate with additional biopsies from suspicious hypervascular foci. Transrectal PDUS guided biopsy should be combined with gray scale TRUS guided biopsy to increase accuracy in the diagnosis of prostate cancer.
Keywords: Biopsy, Cancer, Doppler, Prostate
Özet
Amaç:
Çalışmamızda prostat kanseri tanısında power Doppler Ultrasonografiyi (PDUS) gri skala ultrason(TRUS) ile kıyaslayarak prostat kanseri tanısındaki etkinliğini araştırmayı amaçladık.
Gereç ve Yöntem:
Kliniğimizde 76 olgu power Doppler ultrasonografiile değerlendirilerek rutin 8 kadran ve anormal alanlardan ek biyopsiler yapıldı. Patolojik tanılar benign, kronik prostatit, intraepitelyal neoplazi ve kanser olarak sınıflandırıldı. Olguların b mod ultrasonografi ve power Doppler ultrasonografi bulguları kaydedildi.
Bulgular:
PDUS duyarlılığı, özgüllüğü, pozitif öngörüsel değeri ve negatif öngörüsel değeri sırasıyla %81, %81, %54, %94 olarak bulunmuştur. PDUS’un duyarlılığı ve özgüllüğü TRUS’a göre yüksek bulunmuştur (%43 and % 60 respectively) PDUS kanser olgularını daha yüksek doğrulukla saptamaktadır.
Sonuç:
Prostat Ca tanı ve takibinde gri skala US ve sistematik biopsiler önemli rol oynamaktadır. PDUS biopsi için uygun alanları hipervasküler lezyonlar olarak ortaya koyar. Bu alanlardan alınan ilave biopsiler sonucunda, TRUS ve sistematik biopsi ile kombine bir şekilde kullanıldığında Ca tespit oranını artırır. PDUS eşlikli biopsinin sistematik biopsi ile kombine kullanımı tercih edilmelidir.
Introduction
Prostate cancer has been the most common visceral malignant neoplasm in men since 1984, now accounting for one third of all such cancers [1]. Radiologic examination takes an important role in prostate cancer diagnosis and follow-up. The imaging methods include transrectal ultrasound (TRUS), computed tomography (CT) and magnetic resonance imaging (MRI). It has been demonstrated that TRUS gives more detailed information than either CT or MRI [2].
In spite of a high sensitivity of TRUS, the low positive predictive value (PPV) in the assessment of malignant lesions attenuates its strength. The reason for this low PPV is that hypoechoic lesions in malignant tumors can also be seen in other pathologies. To increase the accuracy, it is necessary to take biopsies from the whole peripheral zone. This has led to the investigation of various methods to decrease the cost and morbidity, while increasing the PPV, to prevent unnecessary biopsies. Lesion size and location, digital rectal examination (DRE) and PSA levels have all been proven to affect TRUS’ PPV [3].
In the early 1990s, the utility of color Doppler ultrasound was recognized for the diagnosis of prostatic lesions as an additive and necessary method to use with gray scale transrectal ultrasound (TRUS) [4]. The role of power Doppler ultrasound (PDUS) in prostate carcinoma has been the subject of several publications [5–6]. PDUS improves the sensitivity of endorectal ultrasound in spite of some false negative results, demonstrates tumor vascularity, detects capsular extension and aids in imaging guided biopsies. The purpose of this study is to demonstrate the beneficial effect of power doppler ultrasound (PDUS) combined with gray scale TRUS guided systematic biopsy.
Materials and Methods
Seventy-six men aged 55–88 years (mean: 68.2 years) with a serum PSA above 4.0 ng/ml [4.02–100] were enrolled in the study. All patients were prescribed ofloxacin 400 mg once a day two days before the biopsy session, for prophylactic purposes. For pain control, a diclofenac suppository and rectal lidocaine were administered 30 minutes and 5 minutes, respectively, before the intervention.
The study was performed using a Toshiba Power Vision 7000 Doppler ultrasound device with a 7 MHz transrectal probe. The patients lay down in the left lateral decubitus position with the knees and hips flexed. For an optimal examination, sufficient gel was spread between the latex cuff and the rectal probe. The whole prostate gland was carefully examined in axial and sagittal sections in all patients. During this examination, the presence of an irregular contour and an asymmetric gland were recorded as abnormal findings.
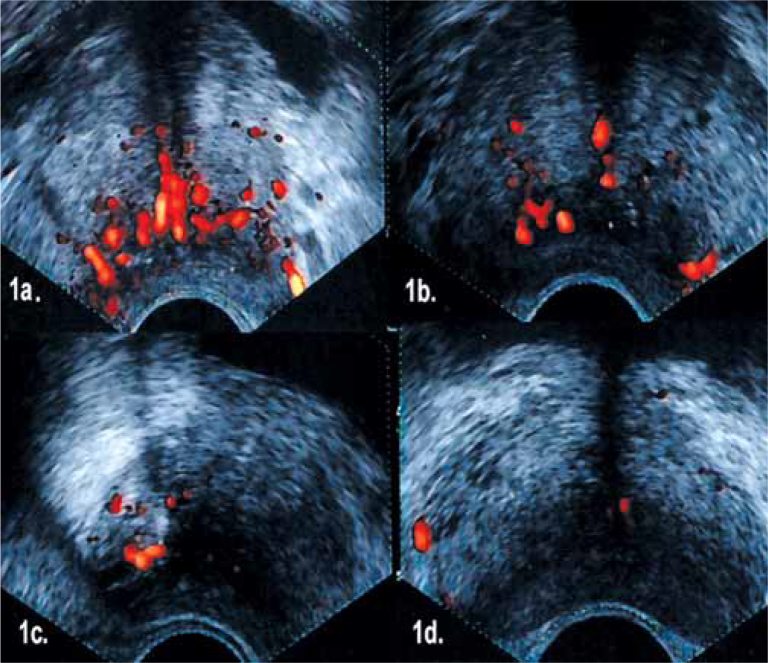
Hypoechoic lesions in the peripheral zones in TRUS were recorded. PDUS gain was set just below the level of background noise. All PDUS images were analyzed and vascularization was graded as below (Fig 1).
0- No abnormal vascularity
1- Low focal vascular clustering
2- Intensive focal vascular clustering
3- Diffuse vascular clustering
Figure 1.
A) Grade 3 (Diffuse flow pattern throughout the gland), B) Grade 2 (Right peripheral zone focally intense flow pattern), C) Grade 1 (Right peripheral zone focally low flow pattern), D) Grade 0 (Scarce flow in the gland).
Grade 0 was considered negative and grades 1, 2 and 3 were considered positive [7].
Figure 1 displays samples from grades 0, 1, 2 and 3.
The transrectal biopsy procedure was initiated after the prostatic echostructure evaluation; an 18 gauge biopsy device was employed for the biopsy. The insertion of the needle to the very proximal region of the targeted lesion was confirmed on the screen. The biopsy-gun was then triggered and a tissue sample was obtained and sent in 10% formaline solution for histologic examination. Additional biopsies included eight core systematic TRUS biopsies (six cores from the peripheral zone, two cores from transitional zone) and, if there were hypoechoic and/or PDUS positive areas, two cores from each. When PDUS displayed diffuse vascular clustering (grade 3), samples were taken from the area where the most intensive signal was seen. When the patients had normal findings on digital rectal examination (DRE), TRUS or PDUS, only eight cores biopsy was performed.
Statistical analysis: The results of the imaging study were correlated with the histologic examinations. A result was declared a true positive if at least one biopsy was positive in the same area as the lesion visualized. A result was declared a true negative if no biopsy was found positive during the examination with no visible lesion. Sensitivity, specificity, and positive and negative predictive values of a cancer diagnosis were calculated. Significance was evaluated using the chi-square test (p=0.05)
Results
The correlation of positive biopsy cores with PDUS and TRUS findings is summarized in Table 1. Six of the 15 hypoechoic hypervascular lesions (40%) were found to be cancerous. Though cancer was only demonstrated in one of 16 hypoechoic non-vascular areas (6%), seven of nine non-hypoechoic hypervascular areas (77%) revealed cancer. PDUS positive areas were determined in 24 cases (31%), of which 13 were malignant and 11 were benign. Sixteen of 76 patients (21%) had prostate adenocarcinoma. According to vascularization grading, 52 cases (68%) were identified as negative (grade 0). Five (6.5%), eight (10%) and eleven (14%) cases were defined as grade 1, grade 2 and grade 3, respectively.
Table 1.
Correlation of cancer positive biopsy cores with PDUS and TRUS findings
| Hypoechoic zone in TRUS (+) | Hypoechoic zone in TRUS (−) | Total | |
|---|---|---|---|
| Hypervascular zone in PDUS (+) | 6/15 (40%) | 7/9 (77%) | 13/24 (54%) |
| Hypervascular zone in PDUS (−) | 1/16 (6%) | 3/36 (8%) | 4/52 (7%) |
| Total | 7/31 (22%) | 9/45 20 (%) | 16/76 (21%) |
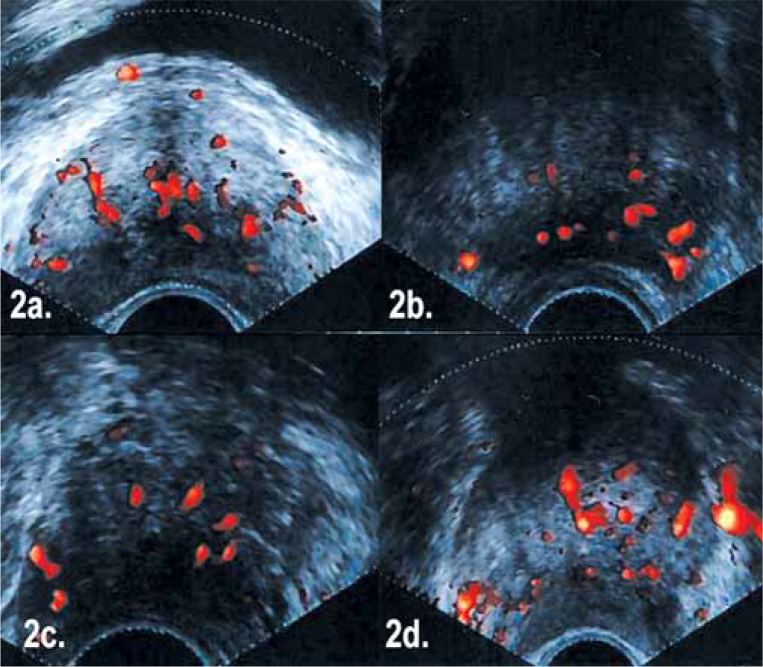
Flow patterns with given histologic diagnoses are depicted in Figure 2. In the 2nd, 3rd and 4th cases no hypechoic TRUS findings were demonstrated, and systematic biopsies gave benign results. However, PDUS examination identified abnormal hypervascular areas in the peripheral zone in all of these cases from where additional biopsies were taken. Histologic examination revealed prostatic adenocarcinoma in these three types of cases. No significant correlation between Gleason scores and PDUS grading was found. Of 11 false positive cases indicated with PDUS, six had a moderate inflammatory reaction and PIN and two had benign prostate tissue. As a result, 13 of 16 adenocarcinoma cases were identified with PDUS and seven were assessed with TRUS. PDUS guided biopsies missed three cases, one of which appeared as a nonvascular hypoechoic nodule in gray scale TRUS. The other two adenocarcinoma cases were neither hypoechoic in gray scale TRUS nor hypervascular in PDUS. The three missed cases were demonstrated with systematic TRUS biopsy. Although 24 cases (31%) were negative with PDUS, 31 cases (40%) were abnormal in gray scale TRUS.
Figure 2.
A) (Grade 3) Glandular diffuse flow pattern (Histologic diagnosis: chronic prostatitis), B) (Grade 3) Left peripheral zone focally diffuse flow pattern (Histologic diagnosis: adenocarcinoma), C) (Grade 1) Right peripheral zone focally low flow pattern (Histologic diagnosis: adenocarcinoma), D) (Grade 2) Right peripheral zone focally intense flow pattern (Histologic diagnosis: adenocarcinoma).
PDUS sensitivity, specificity, positive predictive value (PPV) and negative predictive values were 81%, 81%, 54% and 94%, respectively. PDUS had greater sensitivity and specificity than TRUS (43% and 60%, respectively) and identified cancer cases more accurately.
Discussion
The increasing use of PSA screening over the last few years has resulted in a decrease in the mortality rate from prostate cancer [8]. However, in cases with serum PSA levels from 4.1–10 ng/ml, positive biopsy rates are still low, and unnecessary biopsies cannot be avoided. On the other hand, PSA testing may be responsible for the diagnosis of clinically insignificant prostate cancers sometimes not detected on prostatectomy specimens. In addition, up to 15% of aggressive cancers with Gleason scores ≥7 are not detectable by PSA [9].
Prostate cancer is characterized by increased vascularity compared to normal prostate tissue due to neovascularization and/or growth of the vascular capacity of the existing parenchyma [10].
After color Doppler ultrasound (CDUS) became available, many studies were conducted to assess vascularization in prostate cancer. However, CDUS cannot display weak vessels in small tumors; therefore, PDUS has the advantage over CDUS of showing very low vascular flows. Recent studies have been trying to improve cancer assessment rates with biopsies taken from abnormal vascular foci indicated by PDUS. [11] Sakarya et al. evaluated PDUS in 36 probable prostate cancer cases. They found that PDUS identified suitable biopsy locations [12]. Okihara et al. reported high test performances of PDUS for prostate cancer assessment with a 98% sensitivity and a 99% NPV [13]. Quite recent studies have reported that PDUS is a reliable method for prostate cancer assessment and suggest that it can also predict the aggressiveness of a tumor [14–15].
Halpern et al. evaluated 62 patients with TRUS and PDUS methods, and cancer was demonstrated in 18 patients total. The positive biopsy rate with PDUS was found to be slightly superior to that of systematic biopsy 13% vs 9.7%, respectively [16] Satoru et al. evaluated 108 patients and found that PDUS identified 36 cancer cases while only 32 were identified by gray scale. They also demonstrated that non-hypoechoic hypervascular foci yielded higher rates of cancer than non-vascular hypoechoic foci suggesting that PDUS aids in the identification of additional cancer [7].
In this study, our goal was to evaluate the practical utility and limitation of PDUS guided biopsy by comparing it with gray scale TRUS and systematic TRUS guided biopsy. Six of 15 hypoechoic hypervascular lesions were cancerous (40%) which suggested that biopsies should be taken both from PDUS and gray scale TRUS positive areas. Interestingly, hypoechoic non-vascular areas yielded only 6% positive cancer diagnoses while the hypervascular nonhypoechoic areas yielded 77% positive cancer diagnoses. These findings support the superiority of PDUS over TRUS in the selection of potential biopsy foci and suggest that PDUS guided biopsy has a higher sensitivity and specificity compared to TRUS.
In this study, TRUS and PDUS identified seven and thirteen cancer cases, respectively. PDUS guided biopsy can identify cancers missed by TRUS and sextant systematic biopsy. However, PDUS guided biopsies alone were missed in three of 16 cancer cases diagnosed by a combination of PDUS, TRUS and systematic biopsy. This means that PDUS defines suitable areas for biopsy and increases the cancer identification rate; however, it does not have sufficient accuracy to exclude systematic sextant biopsy.
In a recent study with 105 patients, the relationship between hypervascularity and Gleason scores was investigated. The authors concluded that power Doppler US may contribute to the evaluation of prostate cancer aggressiveness and help direct biopsies to more aggressive foci [14]. In contrast, our study demonstrated no significant correlation between Gleason scores and flow grading. Gleason scores can be high in low focal flow clustering while lesions with intensive focal flow clustering can have low Gleason scores. Among patients with Gleason scores less than 6, only one had grade 1, two had grade 2 and the other two had grade 3 flow patterns. Among patients with Gleason scores greater then 6, two had grade 1, two had grade 2 and four had grade 3 flow patterns. In prostatic inflammation, increased flow ensues at the arteriolar level with inflammatory mediators leading to an increased flow pattern on PDUS with an intensive flow clustering. Both inflammatory alterations and prostate cancer are noticed on PDUS as hypervascular foci. To differentiate cancerous lesions from benign ones, some studies tried to define specific PDUS findings [13–17–18]. For example, Okihara et al. demonstrated that in PDUS positive cases without cancer, prostate volume is significantly higher than PDUS negative cases without cancer. They claimed that the growing inner prostatic tissue needed more blood supply than normal glandular tissue[13]. In a study which evaluated three dimensional PDUS with contrast medium, the sensitivity and specificity were 87% and 79%, respectively, whereas PDUS without contrast had a 77% sensitivity and an 86% specificity [18]. In a retrospective study, 620 radical prostatectomy specimens with preoperative PDUS and TRUS findings were reviewed. The authors reported that PDUS improved the specificity of TRUS for identifying prostate tumors but did not improve the overall accuracy or sensitivity. They also concluded that PDUS could have a role in image guidance for the focal therapy of cancer [19]. Further sophisticated studies should be conducted to demonstrate better cancer assessment with PDUS imaging.
Conclusion
PDUS identifies hypervascular foci as appropriate areas for biopsy. When combined with TRUS and systematic biopsies, additional biopsies from these foci increase the cancer assessment rate. In conclusion, PDUS guided biopsy, without systematic biopsy, seems to have insufficient accuracy. Consequently, it would be better to combine TRUS guided biopsy with PDUS.
Table 2.
Sensitivity, specifity, PPV, NPV of TRUS and PDUS methods
| Sensitivity | Specificity | PPV. | NPV | Accuracy | |
|---|---|---|---|---|---|
| TRUS | 43.75 | 60.00 | 22.58 | 80.00 | 56.58 |
| PDUS | 81.25 | 67.12 | 35.14 | 94.23 | 69.66 |
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer Statistics 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. Erratum in: CA Cancer J Clin. 2005; 55:10–30. [DOI] [PubMed] [Google Scholar]
- 2.Gillenwater JY. Adult and Pediatric Urology. 3rd Edn. I–II. Lippincott; Williams&Wilkins PA,USA: 2002. pp. 1471–654. [Google Scholar]
- 3.Lee F, Torp-Pederson S, Littrup PJ. Hypoechoic lesions of the prostate. Radiology. 1989;170:29–32. doi: 10.1148/radiology.170.1.2462262. [DOI] [PubMed] [Google Scholar]
- 4.Rifkin MD, Sudakoff GS, Alexander AA. Prostate: techniques, results and potential applications of color Doppler US scaning. Radiology. 1993;186:509–13. doi: 10.1148/radiology.186.2.7678467. [DOI] [PubMed] [Google Scholar]
- 5.Nelson ED, Slotoroff CB, Gomella LG, et al. Targeted Biopsy of the Prostate:The Impact of Color Doppler Imaging and Elastography on Prostate Cancer Detection and Gleason Score. Urology. 2007;70:1136–40. doi: 10.1016/j.urology.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Kravchick S, Cytron S, Peled R, et al. Optimal combinations for detection of prostate cancer: systematic sextant and laterally directed biopsies versus systematic sextant and color Doppler-targeted biopsies. J Urol. 2004;63:301–5. doi: 10.1016/j.urology.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S, Yamada Y, Homma Y, et al. Power doppler US-directed prostate biopsy in men with elevated serum PSA levels. Urology. 2002;60:248–52. doi: 10.1016/s0090-4295(02)01702-8. [DOI] [PubMed] [Google Scholar]
- 8.Bossard N, Velten M, Remontet L, et al. Survival of cancer patients in France: a population-based study from the associations of the french Cancer registries (FRANCIM) Eur J Cancer. 2007;43:149–60. doi: 10.1016/j.ejca.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate specific antigen level < or =4,0ng per milliliter. N Engl J Med. 2004;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 10.Frâncu-Doina L, Frâncu LL, Roznovanu D, et al. Évaluation de l’angiogénèse dans les carcinomes prostatiques. Morphologie. 2005;89:211–3. [Google Scholar]
- 11.Wilson NM, Masoud AM, Barsoum HB, et al. Correlation of power Doppler with microvessel density in assessing prostate needle biopsy. Clin Radiol. 2004;59:946–50. doi: 10.1016/j.crad.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Sakarya ME, Arslan H, Unal O, et al. The role of Power Doppler US in the diagnosis of prostate cancer: a preliminary. study. Br. J Urol. 1998;82:386–8. doi: 10.1046/j.1464-410x.1998.00753.x. [DOI] [PubMed] [Google Scholar]
- 13.Okihara K, Kojima M, Nakanouchi T, et al. Transrectal power Doppler imaging in the detection of prostate cancer. BJU Int. 2000;85:1053–7. doi: 10.1046/j.1464-410x.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 14.Boukadoum N, Touati R, Demuynck F, et al. Is power Doppler US a good predictor of prostate cancer aggressiveness? Radiol. 2009;90:299–303. doi: 10.1016/s0221-0363(09)72508-9. [DOI] [PubMed] [Google Scholar]
- 15.Kimura G, Nishimura T, Kimata R, et al. Systematic Sextant Biopsy Versus Power Doppler Ultrasound-guided Target Biopsy in the Diagnosis of Prostate Cancer: Positive Rate and Clinicopathological Features. J Nippon Med Sch. 2005;72:5. doi: 10.1272/jnms.72.262. [DOI] [PubMed] [Google Scholar]
- 16.Halpern EJ, Frauscher F, Strup SE, et al. Prostate: high frequency Doppler US imaging for cancer detection. Radiology. 2002;225:71–7. doi: 10.1148/radiol.2251011938. [DOI] [PubMed] [Google Scholar]
- 17.Franco OE, Arima K, Yanagawa M, et al. The usefulness of power Doppler US for diagnosis prostate cancer: Histological correlation of each biopsy site. BJU Int. 2000;85:1049–52. doi: 10.1046/j.1464-410x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- 18.Unal D, Sedelaar JP, Aarnink RG, et al. Three-dimensional contrast-enhanced power Doppler US and convansional examination methods: The value of diagnostic predictors of prostate cancer. BJU. 2000;86:58–64. doi: 10.1046/j.1464-410x.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg ML, Cowan JE, Carroll PR, et al. The adjunctive use of power Doppler Imaging in the Preoperative Assessment of Prostate Cancer. BJU Int. 2010;105:1237–41. doi: 10.1111/j.1464-410X.2009.08958.x. [DOI] [PubMed] [Google Scholar]