Abstract
Infant botulism is a rare condition caused by intestinal colonization with Clostridium botulinum. The enteric toxin causes intestinal immobility and progressive descending paralysis due to the effect on acetylcholine release at the neuromuscular junction and other cholinergic nerve terminals, particularly in the gut. Herein, we report an infant with infantile botulism because of rare clinically entity, with early diagnosis and appropriate treatment recover no squeal.
Keywords: Botulism, Clostridium botulinum, Infant pitosis
Özet
İnfant botilismus Clostridium botulinum ile barsakta kolonizasyon sonucu meydana gelen nadir bir klinik durumdur. Bu enterik toksin barsağa ulaştığında öncelikle intestinal motiliteyi durdurur, daha sonra ise başta barsak olmak üzere nöromuskuler kavşaktaki asetilkolin salınımına neden olarak ilerleyici, aşağıdan başlayan yukarıya doğru ilerleyen paraliziler meydana getirir. Burada çok nadir rastanan bir infantil botilismus vakası sunulmaktadır. Erken tanı ve uygun tedavinin sekelsiz iyileşmede önemi vurgulanmaktadır.
Introduction
Infant botulism is a rare condition caused by intestinal colonization with Clostridium botulinum. The enteric toxin causes intestinal immobility and progressive descending paralysis due to the effect on acetylcholine release at the neuromuscular junction and other cholinergic nerve terminals, particularly in the gut. Infant botulism was described in 1933 but at the time was misclassified as hypotonia of other etiology [1]. In 1976, Pickett et al. [2]. described the first case of infant botulism, and since then, many cases and surveys have been collected and reported. Infant botulism is recognized as the most frequent form of human botulism in the United States. In contrast to food-borne botulism, in which preformed toxin is ingested in a single episode, in infant botulism, there is continued intra-intestinal production of toxin due to clostridial colonization of the large intestine. The infant intestinal tract lacks protective bacterial flora and Clostridium-inhibiting bile acids, which allows the C. botulinum to flourish and produce the toxin that causes disease. Although about 98% of affected infants present between 1 and 6 months of age, infant botulism is reported as early as the first week of life [3] and as late as 12 months of age. Although isolated cases of C. butyricum type E and C. baratii type F have been reported, C. botulinum types A and B primarily cause infant botulism [4].
Herein, we report a rare case of infantile botulism, with early diagnosis and appropriate treatment resulting in no sequela.
Case Report
A 5-month-old, previously healthy female was admitted with an 8-day history of constipation, 4 days of poor sucking, decreased limb movements and ptosis (Figure 1). The infant and other family members had been fed a small amount of grape molasses for 4 weeks; however, the other family members were asymptomatic. The patient had been seen at an outside emergency department and given fluid boluses and ceftriaxone for urinary tract infection 1 day before transfer to our hospital. Her prenatal, natal and postnatal histories were unremarkable. She was born at 39 weeks gestational age and at a birth weight of 4500 g. Delivery was uncomplicated. Neurodevelopmental milestones were normal. She was the second infant of the family and there was no consanguinity between the parents. There was no family history of neuromuscular disorders or dysmorphic syndromes.
Figure 1 and 2.
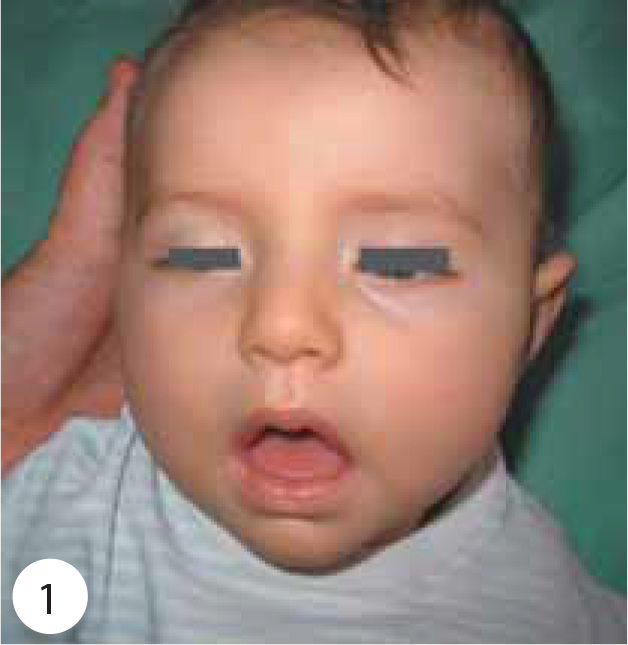
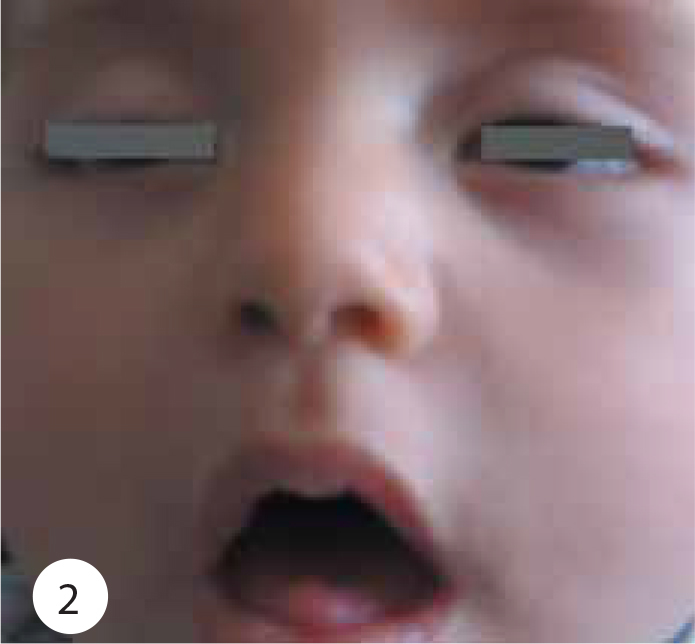
Shows ptosis, facial weakness, generalized hypotonia.
On physical examination, her weight was 7.400 g (75–90 percentile), height 69 cm (90–97 percentile) and head circumference 42.5 cm (50–75 percentile). Body temperature, pulse rate, respiratory rate and systolic arterial pressure were 37°C, 100 beats/min, 20/min and 90 mmHg, respectively. Moderate general condition, severe hypotonia and hypo-responsiveness were observed. She had a weak cry, poor suck, diminished gag reflex, difficulty swallowing, ptosis, sluggish pupillary light reflexes, ophthalmoplegia, facial weakness, diminished head control with neck and shoulder-girdle weakness, generalized hypotonia, symmetric flaccid paralysis of the skeletal muscles, reduced spontaneous movement, and absent muscle stretch reflexes (Figures 1 and 2). She had no head control and she could not sit without support (Figure 3). The remainder of the physical findings was normal.
Figure 3.
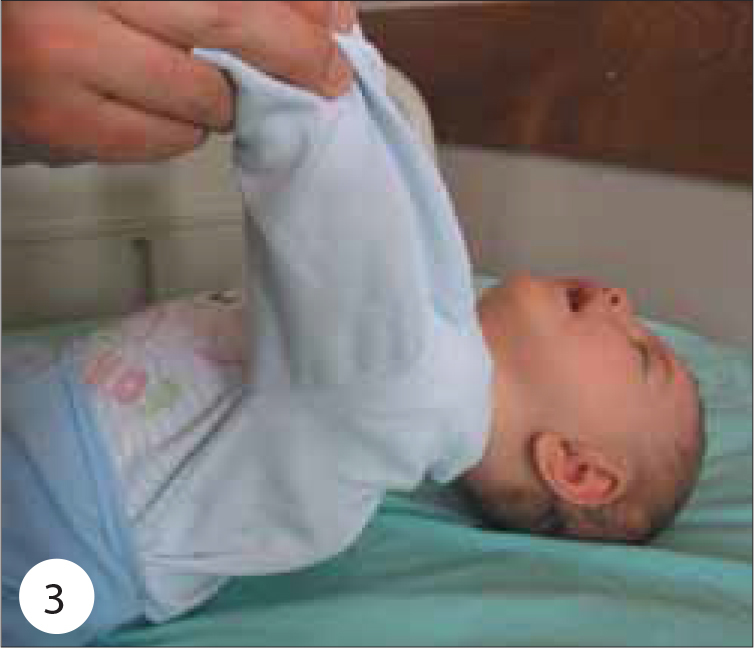
She had no head control and generalized hypotonia.
On laboratory investigation, hemogram, serum electrolytes, renal and liver function tests, erythrocyte sedimentation rate and C-reactive protein level were normal. Blood gas analysis was also normal. Cerebrospinal fluid examination showed no cell, protein 22.8 mg/dL, glucose 52.3 mg/dL, and chloride 121.3 mEq/L. Urinary analysis was unremarkable. No microorganism was isolated from cerebrospinal fluid and urine cultures. Electroencephalography and brain MRI were normal.
On the electromyography (EMG) performed on the patient, no pathological decrement or increment was detected in the repetitive stimulations at 3,5,10,20 and 30 Hz, which were stimulated through the right nervus ulnaris and recorded through the right musculus abductor digiti minimi. Motor and sensory conduction and sensory conductions studied with concentric needle EMG were within normal limits in the right upper and lower extremities. Motor conduction studies revealed normal values, but motor action potential values were found to be low. An F answer could not be obtained. Rarefaction of the MUP (motor unit potential) activity has attracted attention in concentric needle EMG. The findings were in accordance with motor axonal neuropathy.
Respiration was not affected and neither respiratory support nor supplementary oxygen was needed. She was given supportive treatment and was treated symptomatically with intravenous fluids and enteric tube feeding. First, we considered Guillain-Barre syndrome and administered IVIG 1 g/kg/day for 2 days. In addition, botulism immune globulin was not administered. Oral feeds were initiated 7 days later. Fourteen days after supportive treatment she was found to have improved slightly with decreasing ptosis and increasing muscular tonus and cry, and she was discharged home with her family.
Two days after admission, she was reevaluated and intestinal (infant) botulism was suspected. However, botulism acquired from consumption of food with preformed neurotoxin could not be excluded. She has been followed for 6 months with no sequela.
Discussion
Since it was first described as a recognizable and discrete entity in 1976, infant botulism has become the most frequently reported syndrome related to botulinum toxin, accounting for roughly 75% of all annual cases in the United States [2, 5]. The United States Centers for Disease Control and Prevention (CDC) reviewed the reported cases of botulism in all of its forms in the United States between 1899 and 1996 and found that 1442 cases of infant botulism were reported in 46 states between 1976 and 1996 [6]. Type A infections accounted for 46.5% and type B for 51.9% of those reported. This is strikingly similar to the 96 cases reported to the CDC in 2005, of which 46% were type A and 54% were type B [7]. Since reporting began to stabilize in 1980, the average annual incidence of infant botulism in the United States is approximately 1.9/100 000 live births.
The clinical syndrome of infant botulism usually occurs between 2 weeks and 1 year of age, with a median age of about 10 weeks [5]. The hypothesized incubation period for the development of infant botulism in children exposed to botulinum spores is 3 to 30 days, although the cases mentioned above illustrate that this period may be shorter with some forms of Clostridia [8]. The syndrome is usually characterized by a history of constipation, followed by subacute progression over 4 to 5 days of bulbar and extremity weakness often followed by respiratory compromise and associated medical complications. Our patient’s constipation began approximately 8 days prior to presentation. Her passive behavior, ptosis and weak suck began approximately 4 days prior to presentation. Typically, complete resolution occurs over the ensuing weeks to months with supportive care, although relapses, poor clinical outcomes, and death have been reported in rare instances [9]. Fourteen days after supportive treatment, our patient was found to have improved slightly with decreasing ptosis and increasing muscular tonus and cry, and she was discharged home with her family. Based on a broad range of case reports, infant botulism may represent a spectrum of varying clinical presentations and outcomes, encompassing “asymptomatic carriers” of C. botulinum at one end of the spectrum, and patients succumbing to rapid progression and death at the other [10].
The symptoms described in infant botulism are related to peripheral cholinergic blockade due to botulinum toxin. These include constipation or a change in stool pattern with decreased frequency of bowel movements related to autonomic dysfunction; bulbar weakness manifesting as hypophonia and a weak cry, poor suck, and diminished gag, leading to difficulty swallowing, drooling, and often dehydration; cranial nerve palsies such as ptosis, sluggish pupillary light reflexes, ophthalmoplegia, and facial weakness; diminished head control with neck and shoulder-girdle weakness, then progressing to generalized hypotonia; and a descending, symmetric flaccid paralysis of the skeletal muscles with reduced spontaneous movement, delayed motor developmental milestones, and diminished or absent muscle stretch reflexes. Other findings of autonomic dysfunction may result, including anhydrosis, dry mouth and throat, often leading to mucosal irritation and an incorrect initial clinical suspicion for pharyngitis, hypertension alternating with postural hypotension, brady- and tachyarrhythmias, flushing, decreased anal sphincter tone, and bladder atony. Respiratory compromise, hypoventilation, hypoxia, and the need for mechanical ventilation in a majority of cases ensue, as the diaphragmatic and accessory respiratory musculature becomes affected [11]. Our patient had constipation, passive behaviors, ptosis, weak suck, diminished gag, difficulty swallowing, sluggish pupillary light reflexes, ophthalmoplegia, facial weakness, and diminished head control with neck and shoulder-girdle weakness. She progressed and experienced generalized hypotonia; descending, symmetric flaccid paralysis of the skeletal muscles with reduced spontaneous movement; delayed motor developmental milestones; and diminished muscle stretch reflexes. However, she had no findings of autonomic dysfunction.
Botulinum toxin does not cross the blood-brain barrier, and therefore does not exert central nervous system effects. As a result, mentation is generally unaffected, unless there are significant secondary complications, such as hypoxemia, cardiorespiratory failure, hypoglycemia, profound dehydration or electrolyte disturbances. The time course of serious complications from the onset of symptoms often ranges from less than 24 hours to greater than 1 week [12]. Our patient did not experience any of these complications. She had a weak smile to mother’s stimulus.
The differential diagnosis of an infant who initially presents with hypotonia includes sepsis, meningitis, tick paralysis, Guillain-Barre syndrome, myasthenia gravis, metabolic derangements such as hypocalcemia and hypernatremia, hypothyroidism, inborn errors of metabolism, myopathies, spinal muscular atrophy, poliomyelitis, organophosphate poisoning, and heavy metal poisoning [11]. Our patient had did not have a metabolic disorder, sepsis, meningitis, tick paralysis, or hypothyroidism. She had no history of organo-phosphate poisoning or heavy metal poisoning. CPK, ALT, AST were normal. Initially, we considered Miller-Fisher variant of Guillain-Barré syndrome and gave IVIG 1 g/kg/day for 2 days. Subsequently, we reevaluated the patient. The patient was diagnosed with infant botulism because she had constipation, facial diplegia, normal cerebrospinal protein and descending muscle weakness.
Honey ingestion has been long felt to be a risk factor for infant botulism in the United States. In their review of cases in Utah in the late 1970s, Thompson et al. found that 10 of the 12 infants had ingested honey prior to symptom onset [10]. Seven of these 10 subsequently had samples of honey and other food sources from their homes cultured. In two of these seven, clostridial organisms, but not their toxins, were detected in honey specimens fed to infants who acquired the disease. One type A. C botulinum specimen and one type B specimen were isolated. The organism was not isolated from a variety of other commercially prepared or home-canned foods fed to these infants [11].
Our patient had not eaten honey. However, she had eaten grape molasses for 4 weeks. Grape molasses ingestion can cause botulism. The diagnosis of infant botulism is, largely, a clinical one. It is characterized by normal laboratory values and spinal fluid analyses and typical features on electrodiagnostic testing. Imaging and electroencephalography are usually normal, barring major hypoxic events. Edrophonium testing is normal. Isolation of the causative organism or detection of its toxin in fecal materials obtained from affected infants may support the diagnosis, with each detectable for up to 4 months after the onset of symptoms and well into the recovery phase. A toxin neutralization mouse bioassay performed on stool filtrate at a state lab or by the CDC is the only reliable confirmatory test for infant botulism [13]. Electro-diagnostic studies, in particular EMG, are usually highly reliable in the diagnosis of infant botulism, but can have false positive results [14]. Nerve conduction studies are usually normal, while there may be a characteristic EMG pattern of increased insertional activity and brief-duration, small-amplitude, overly abundant motor-unit action potentials [13]. These findings are consistent with acute denervation but are not specific to botulism and can be seen in axonal neuropathies and certain myopathic processes, as well [5]. M-wave amplitudes are small, with incremental responses seen with paired nerve stimuli at short intervals or rapid repetitive nerve stimulation, and decremental responses with paired nerve stimuli at long intervals or slow repetitive nerve stimulation [15]. Single-fiber EMG may reveal increased jitter or the presence of blocks in neuromuscular transmission, though results may be variable and nonspecific in severe cases. If initial EMG is negative, and the clinical suspicion for infant botulism remains high, a follow-up study may be considered [16]. Our patient’s EMG did not reveal any pathologic increments or decrements on two independent exams.
Despite the relative rarity of infant botulism, this case illustrates the importance of maintaining a high level of clinical suspicion in hypotonic infants presenting for emergency medical attention. Infant botulism is highly treatable, and prompt diagnosis is essential. Given the excellent outcome of infant botulism, early and aggressive attention to respiratory and nutritional support is essential.
Footnotes
Conflict interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Arnon SS, Damus K, Chin J. Infant botulism: epidemiology and relation to sudden infant death syndrome. Epidemiol Rev. 1981;3:45–66. doi: 10.1093/oxfordjournals.epirev.a036239. [DOI] [PubMed] [Google Scholar]
- 2.Pickett J, Berg B, Chaplin E, Brunstetter-Shafer MA. Syndrome of botulism in infancy: clinical and electrophysiologic study. N Engl J Med. 1976;295:770–2. doi: 10.1056/NEJM197609302951407. [DOI] [PubMed] [Google Scholar]
- 3.Nevas M, Lindström M, Virtanen A, et al. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol. 2005;43:511–3. doi: 10.1128/JCM.43.1.511-513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armada M, Love S, Barrett E, Monroe J, Peery D, Sobel J. Foodborne botulism in a six-month-old infant caused by home-canned baby food. Ann Emerg Med. 2003;42:226–9. doi: 10.1067/mem.2003.259. [Erratum in Ann Emerg Med. 2003; 42:600]. [DOI] [PubMed] [Google Scholar]
- 5.Fox CK, Keet CA, Strober JB. Recent advances in infant botulism. Pediatr Neurol. 2005;32:149–54. doi: 10.1016/j.pediatrneurol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Botulism in the United States. 1899–1996 [Google Scholar]
- 7.Centers for Disease Control and Prevention . Surveillance for Botulism, Summary of 2005 Data. Atlanta, GA: Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 8.Domingo RM, Haller JS, Gruenthal M. Infant Botulism: Two Recent Cases and Literature Review. Journal of Child Neurology. 2008;23:1336–46. doi: 10.1177/0883073808318200. [DOI] [PubMed] [Google Scholar]
- 9.Glauser TA, Maguire HC, Sladky JT. Relapse of infant botulism. Ann Neurol. 1990;28:187–9. doi: 10.1002/ana.410280214. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JA, Glasgow LA, Warpinski JR, Olson C. Infant botulism: clinical spectrum and epidemiology. Pediatrics. 1980;66:936–42. [PubMed] [Google Scholar]
- 11.Francisco AM, Arnon SS. Clinical mimics of infant botulism. Pediatrics. 2007;119:826–8. doi: 10.1542/peds.2006-0645. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell WG, Tseng-Ong L. Catastrophic presentation of infant botulism may obscure or delay diagnosis. Pediatrics. 2005;116:436–8. doi: 10.1542/peds.2005-0297. [DOI] [PubMed] [Google Scholar]
- 13.Long SS. Infant botulism. Pediatr Infect Dis J. 2001;20:707–9. doi: 10.1097/00006454-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JA, Filloux FM, Van Orman CB, et al. Infant botulism in the age of botulism immune globulin. Neurology. 2005;64:2029–32. doi: 10.1212/01.WNL.0000166950.35189.5E. [DOI] [PubMed] [Google Scholar]
- 15.Cherington M. Electrophysiologic methods as an aid in diagnosis of botulism: a review. Muscle Nerve. 1982;5:28–9. [PubMed] [Google Scholar]
- 16.Schreiner MS, Field E, Ruddy R. Infant botulism: a review of 12 years’ experience at the Children’s Hospital of Philadelphia. Pediatrics. 1991;87:159–65. [PubMed] [Google Scholar]


