Abstract
Objective:
Although bone mineral density (BMD) is an important predictor of hip fracture, there is a large overlap of BMD values between those who fracture their hips and those who do not. The aim of this study was to evaluate differences in the structural parameters of the hip in patients with osteopenia and osteoporosis in the hip region and to assess their relationship with osteoporotic fracture risk, age and gender.
Materials and Methods:
In this observational retrospective study, 150 patients with osteopenia (100 postmenopausal women and 50 men ≥50 years of age) and 125 patients with osteoporosis in the hip (100 postmenopaussal women and 25 men ≥50 years of age) were included. In addition to densitometry measurements by DEXA (Dual Energy X-ray Absorbimetry), structural variables were determined using the Hip Strength Analysis program (HSA).
Results:
In logistic regression analyses, the femoral neck BMD (odds ratio (OR), 2.6; 95% Confidence Interval (CI) 1.8–3.8), age (OR per 10 years 1.4; 95% CI, 1.1–1.9), femoral neck shaft angle (NSA) (OR 1.5; 95% CI, 1.2–2.1), Femur Strength Index (FSI) (OR 1.6; 95% CI 1.3–2.2), and Cross sectional area (CSA) (OR 1.6; 95% CI 1.2–2.1) were all associated with osteoporotic fractures in women and men. Osteopenic patients had smaller femoral neck-shaft angles (NSA) compared to osteoporotic patients (p<0.05). This angle was larger in women (p<0.05); and women had decreased (FSI) (p<0.001) and CSA (p<0.05), which cause increased fracture risk.
Conclusion:
Spatial distribution of bone tissue is a useful determinant of fracture risk.
Keywords: Osteoporosis, hip geometry, fracture risk, BMD
Özet
Amaç:
Kemik mineral dansitometrisi femur kırık riskini önceden tahmin etmede önemli bir belirleyici olmasına rağmen kalça kırığı olan ve olmayan kişilerin kemik mineral dansitometri değerleri büyük farklılıklar göstermektedir. Bu çalışmanın amacı, kalça bölgesinde osteopeni ve osteoporoz olan hastalarda kalça yapısal parametrelerindeki değişimi tespit etmek ve aynı zamanda bu değişikliklerin osteoporotik kırık riski, cinsiyet ve yaşla olan ilişkisini araştırmaktı.
Gereç ve Yöntem:
Bu gözlemsel retrospektif çalışmada, kalça bölgesinde osteopenisi olan 150 hasta (100 postmenapozal kadın ve 50 erkek ≥50 yaş) ve osteoporozu olan 125 hasta (100 postmenapozal kadın ve 25 erkek ≥50 yaş) çalışmaya alındı. DEXA ile yapılan dansitometri ölçümleri yanı sıra kalça yapısal analiz programı da kullanıldı ve geometrik değişkenler belirlendi.
Bulgular:
Regresyon analizinde, kalça BMD (femur boynu için odds ratio (OR), 2.6; %95 güven aralığı (CI) 1.8–3.8), yaş (OR her 10 yıl 1.4; %95 CI, 1.1–1.9), femur boyun şaft açısı (OR 1.5; %95 CI, 1.2–2.1), Femur Kuvvet Indeksi (FSI) (OR 1.6; %95 CI 1.3–2.2), Çapraz kesit alanı (CSA) (OR 1.6; %95 CI 1.2–2.1) hem kadın hem de erkeklerde osteoporotik kırıklar ile ilişkiliydi. Osteopenik hastalarda femur boyun-şaft açısı osteoporotik hastalara kıyasla daha küçüktü (p<0.05). Bu açı kadınlarda erkeklerden daha büyük bulundu (p<0.05), aynı zamanda kadınlarda FSI ve CSA daha azdı (p<0.05), bu bulgular artmış kalça kırığı riski ile ilişkilidir.
Sonuç:
Kırık riskini belirlemede kemik dokunun yapısal dağılımı önemlidir.
Introduction
Although there is a significant correlation between BMD at the femoral neck and the mechanical strength of the femur [1, 2], BMD itself is only one component of bone strength. Bone strength is determined not only by the amount of bone, but also by age-related changes in the spatial distribution of bone tissue, which is an important determinant of fracture risk that is not captured completely by BMD alone.
Structural geometry determines the way that stress produced by loading forces is transmitted through the bone. A growing body of evidence indicates that bone geometry contributes substantially to bone strength and fracture risk [3, 4]. Simple increases in bone mass do not necessarily increase load bearing capacity significantly. Geometric properties, such as the distribution of bone mass and the length and angle of the femoral neck, can be used to calculate the amount of stresses on the bone. BMD is an important predictor of hip fracture [5, 6], but there is a large overlap in BMD values between those who fracture their hips and those who do not [7–9]. Aging and osteoporosis each alter the distribution and the amount of bone tissue in the hip, and it seems likely that geometric effects underlie the good predictive ability of BMD. The hip structural analysis (HSA) method has been useful in exploring this issue, because the mineral data at a particular bone cross-section is thereby expressed in geometric terms [10–13].
A recent review pointed out that osteoporosis is underdiagnosed [14]; patients discharged from the hospital after hip fracture are commonly not diagnosed or treated for osteoporosis, although the risk of future fracture is very high. The Fracture Risk Assessment Tool (FRAX) estimates the 10-year probability of fracture on the basis of clinical risk factors for fracture and bone mineral density, and clinical risk factors predict fracture risk better than either alone [15, 16]. Because approximately one half of patients who have fragility fractures do not have T scores in the osteoporosis range [14, 17, 18], it would be clinically valuable to be able to identify the subset of osteopenic patients who are candidates for the treatment. Because age is an important risk factor for fracture that is independent of BMD, older women with osteopenia should be treated with medication for osteoporosis [14].
Sideways falls precipitate most elderly hip fractures, and the impact causes a combination of bending and axial compression in the proximal femur. Bending leads to axial compressive stresses superiorly and tensile stresses inferiorly (the reverse effect to that of stance). The maximum stress in a cross-section under bending is determined by the section modulus, and it has been hypothesized that this parameter should explain the predictive ability of BMD. In the Rotterdam study, section moduli were indeed significantly smaller in 106 female hip fracture patients before fracture than in controls [11]. Femoral geometry may be assessed noninvasively by DXA-based HSA. The technique of HSA provides measures of bone size and mineral distribution that allow for indirect evaluation of environmental influences on bone mechanical properties [19].
The aim of this study was to assess the changes in the hip structural parameters of osteopenic and osteoporotic patients and also to assess their relationships with age, gender and fracture risk.
Materials and Methods
In this retrospective study, the BMD measurement records of patients who had been treated at the osteoporosis clinic were investigated. The selection criteria included patients whose BMD measurements were performed using the same machine and evaluated by the same operator. The aim was to standardize the data as much as possible. 150 patients with osteopenia (100 postmenopausal women and 50 men ≥50 years of age) and 125 patients with osteoporosis in the hip (100 postmenopaussal women and 25 men ≥50 years of age) were included.
In addition to the conventional densitometry measurements by Lunar DPX DXA system (GE Healthcare), structural variables were determined by using the hip sutructural analysis program (HSA). We measured structural parameters using the HSA program and evaluated their association, compared with standart hip BMD, with osteoporotic fracture risk in a population based sample.
Fractura ascertainment
Subjects were interviewed in accordance with a standart protocol in order to collect clinical data, including a comprehensive fracture history.
The diagnosis of vertebral fracture was accepted on the basis of the presence of compression, wedging or collapse in one or more thoracic or lumbar vertebrae. The subset of “osteoporotic” fractures was defined as clinically recognized fractures of the hip, spine or distal forearm that resulted from minimal or moderate trauma (eg. A fall from standing hight or less) among persons over 35 years of age (20).
HSA
BMD (g/cm2), BMC (g) and the area (cm2) of the femoral neck were determined using the manufacturer’s analysis software. The HSA program was used for analysis of the structural variables. This program automatically calculates a number of bone geometry variables from the scan image and bone distribution variables derived from information contained within DXA X-ray absorption curves [19]. The following variables are presented in Figure 1:
Cross-Sectional Moment of Inertia (CSMI): A measure of the distribution of material around the next axis used to calculate the resistance to bending.
Cross-Sectional Area (CSA): The minimum CSMI section within the neck region of interest. In mechanical terms, CSA is an indicator of resistance to a load directed along the bone axis.
d1: Distance from the center of the femoral head to the minimum CSMI.
y: Distance from the center of mass to the superior neck margin of the section corresponding to the minimum CSMI.
Ø Angle: Femoral neck angle.
Hip axis length (HAL): The distance measured along the neck axis from the base of the greater trochanter to the inner pelvic rim.
Femur Strength Index (FSI): The ratio of the estimated compressive yield strength of the femoral neck to the expected compressive stress of a fall on the greater trochanter adjusted for the patient’s age, height and weight; it is calculated using the parameters mentioned above (1 to 5). The greater the FSI, the lower the risk of hip fracture from a fall on the greater trochanter.
Figure 1.
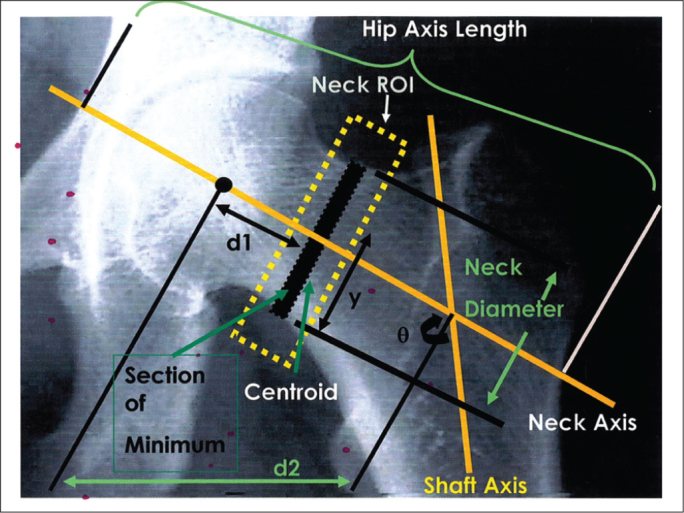
Hip structural parameters.
The section modulus (Z) is an indicator of the ability of bone to resist bending and torsion. The section modulus is also an estimate of the ability of the femoral neck to withstand bending forces, and it is computed as the CSMI divided by half the width of the femoral neck.
Statistical analysis
The Kolmogorov-Smirnov test was used to confirm that data were normally distributed. Continuous variables were summarized as arithmetic mean and standard deviation (SD).
The unpaired samples t-test was used to analyze the statistical differences in the CSMI, CSA, Z modulus, Femoral Strength Index, HAL, Ø angle, femoral neck angle and d1 between osteopenic and osteoporotic patient groups.
A multiple linear regression analysis was performed to detect independent predictors of the CSMI and to find confounding effects between potentially independent predictors (age, BMI, gender and hip BMDs). A variable was entered into the model if the probability of its statistical score was less than the entry value (0.05) and was removed if the probability was greater than the removal value (0.1). A stepwise method was used to construct multiple linear regression models. This regression analysis was also applied for CSA, Z modulus, Strength index, HAL, teta, femoral neck angle. A p value of less than 0.05 was considered statistically significant.
The relative risk of various fractures was estimated by odds ratios (OR) obtained from multiple logistic regression models wher fracture was the dependent variable and age, gender, BMD and structural variables were the potential predictors. Variables were selected in a stepwise fashion. The data management software package used was the PASW Statistic 18.
Results
Altogether, 40 postmenopausal women (20%) and 10 men (14%) ≥50 years of age had one or more “ osteoporotic” fractures as defined in “ Methods”.
In logistic regression analyses, the hip BMD (odds ratio (OR), 2.6; 95% Confidence Interval (CI) 1.8–3.8 for femoral neck BMD), age (OR per 10 years 1.4; 95% CI, 1.1–1.9), femoral neck shaft angle (OR 1.5; 95% CI, 1.2–2.1), Femur Strength Index (FSI) (OR 1.6; 95% CI 1.3–2.2), Cross sectional area (CSA) (OR 1.6; 95% CI 1.2–2.1) were all associated with osteoporotic fractures in women and men.
Osteopenic patients had smaller femoral neck-shaft angles compared to osteoporotic patients (p<0.05). Femoral neck angle increased with age. This angle was larger in women (p<0.05) compared to men, which means that women are in greater risk for the hip fracture.
Women also had decreased femur strength ındex (FSI) (p<0.001). The lower the FSI, the higher the hip fracture risk from a fall on the greater trochanter. Decreased CSA (p<0.05) in women indicates a lower resistance to loads directed along the bone axis.
In a separate analysis that combined men and women, male gender was associated with a lower risk of osteoporotic fracture (OR, 0.6; 95% CI, 0.4–0.9).
Discussion
Bone strength is determined not only by the amount of bone mineral, but also by its spatial distribution with respect to the loading forces that may be encountered. Age and gender have well known effects on bone health. The conventional wisdom about age-related geometric remodeling of cortical bones is that loss of bone mineral and cortical endosteal expansion can be compensated by subperiosteal expansion. This type of adaptation of cortical bone can maintain the bending rigidity of long bones in males but not in females because bone mineral loss is too fast and too great in females [19]. This contributes to the greater fracture incidence in women. Additionally, the center of mass of the cross section of the femoral neck appears to move closer to the calcar region with age because of a greater decrease in BMD in the cranial half of the femoral neck [19]. This geometric change in the femoral neck increases the stress on the femoral neck, particularly on its tensile surface. The magnitude of these changes is nearly twice as great in females when compared with males, which may contribute to the higher incidence of fracture of the femoral neck in females [19].
During aging, periosteal bone formation is slowed, but the process continues in women and men; additionally, with endocortical resorption, it results in the widening of both periosteal and endocortical diameters. However, periosteal widening occurs more slowly than endocortical widening, leading to increased cortical thinning and susceptibility to local buckling [21]. Because of the sexual dimorphism in bone geometry, this structural instability may occur earlier in life in women than men [21, 22].
Comparisons across age groups of women revealed a consistent and progressively worsening pattern of hip structural geometry with increasing age. Women age 85 years or older had the most unfavorable features, but the changes were already apparent at age 75. No strong age trends in the section moduli at either the femoral neck or the intertrochanteric region were observed, which is consistent with the observation that expansion of the outer diameter of the bone tends to preserve resistance to pure bending [23, 24]. However, expansion of the outer diameter, combined with cortical thinning, indicates that the compensatory changes are accomplished with progressively less bone. A wider bone requires less bone tissue for the same section modulus, which causes an increase in susceptibility to local cortical buckling. Among women, femoral neck fractures from low trauma occur more frequently than trochanteric fractures before age 60, but increasing numbers of trochanteric fractures occur in older women [25]. In one study, elderly women showed significant age-associated increases in the buckling ratio at both the femoral neck and the intertrochanteric region, suggesting that strength is compromised on both sites [26].
The accelerated rates of hip fracture after age 80 may be partly explained by an unfavorable distribution of bone tissue and the ensuing increased bone fragility. These structural changes are consistent with imbalances in processes of bone modeling and remodeling, including periosteal apposition and endocortical resorption. Therapeutic agents should target the cellular aspects of bone formation and destruction to maintain or restore the tissue.
The importance of age and gender can also be seen using the FRAX model, which is an electronic clinical tool (www.shef.ac.uk/FRAX/) for calculating fracture risk on the basis of the bone mineral density of the femoral neck, the patient’s age, gender, height and weight, and seven clinical risk factors, including previous fracture, having had a patient who had a previous hip fracture, current smoking, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, and ingestion of three or more units of alcohol daily [15]. When this information is entered together with the brand of DXA machine used (Hologic, GE Lunar or Norland), the algorithm estimates the 10-year probability of a major osteoporotic fracture (hip, spine, proximal humerus or distal forearm) and the 10-year probability of hip fracture [14, 15].
Results of our study revealed that age has a strong effect on BMD, as it was noticed that patients with osteoporotic hips were significantly older than patients with osteopenic hips.
Statistically significant increase in femoral neck-shaft angle was apparent as a result of aging. Osteopenic patients had smaller femoral neck-shaft angles compared to osteoporotic patients. This angle was also larger in women when compared to the men, indicating the effect of gender. As documented in several studies a larger neck-shaft angle is one of the most frequently described measurements that have been associated with an increased risk of fracture [27, 28]. In our study, women also had decreased FSI, CSA and CSMI when compared to men, which means that women again are at bigger risk for hip fractures when compared to men. Estimated from the CSMI value, BMD was reported to have nearly 50 % effect on bone strength, indicating that CSMI includes substantial information about strength not contained in the BMD measurement alone.
In conclusion, the strength of bones is determined not only by the amount of bone but also by age-related changes in the spatial distribution of bone tissue which is an important determinant of fracture risk that is not captured completely by BMD and hip structural analysis provides better information.
Table 1.
Differences between osteopenic and osteoporotic women in hip structural parameters
| Femoral Neck BMD | n | mean | Std Deviation | p | |
|---|---|---|---|---|---|
| Age | Osteopenic | 100 | 58.94 | 8.23 | 0.000* |
| Osteoporotic | 100 | 69.25 | 8.36 | ||
| BMI | Osteopenic | 100 | 28.27 | 4.53 | 0.18 |
| Osteoporotic | 100 | 26.25 | 4.76 | ||
| Femoral neck BMD | Osteopenic | 100 | 0.76 | 0.05 | 0.000* |
| Osteoporotic | 100 | 0.61 | 0.06 | ||
| Trochanter BMD | Osteopenic | 100 | 0.63 | 0.07 | 0.000* |
| Osteoporotic | 100 | 0.54 | 0.06 | ||
| FSI | Osteopenic | 100 | 1.26 | 0.33 | 0.08 |
| Osteoporotic | 100 | 1.13 | 0.27 | ||
| CSMI | Osteopenic | 100 | 6.79 | 2.11 | 0.01* |
| Osteoporotic | 100 | 5.94 | 2.17 | ||
| CSA | Osteopenic | 100 | 107.67 | 13.54 | 0.000* |
| Osteoporotic | 100 | 86.03 | 14.99 | ||
| HAL | Osteopenic | 100 | 35.59 | 7.87 | 0.44 |
| Osteoporotic | 100 | 34.23 | 11.21 | ||
| NSA | Osteopenic | 100 | 127.2 | 0.19 | 0.05* |
| Osteoporotic | 100 | 128.3 | 0.36 |
p<0.05 represents statistically significant results (*)
Table 2.
Differences between osteopenic and osteoporotic men in hip structural parameters
| Femoral Neck BMD | n | mean | Std Deviation | p | |
|---|---|---|---|---|---|
| Age | Osteopenic | 50 | 60.94 | 8.44 | 0.000* |
| Osteoporotic | 25 | 69.75 | 8.16 | ||
| BMI | Osteopenic | 50 | 29.17 | 4.13 | 0.18 |
| Osteoporotic | 25 | 28.25 | 4.26 | ||
| Femoral neck BMD | Osteopenic | 50 | 0.76 | 0.05 | 0.000* |
| Osteoporotic | 25 | 0.68 | 0.02 | ||
| Trochanter BMD | Osteopenic | 50 | 0.6 | 0.07 | 0.000* |
| Osteoporotic | 25 | 0.57 | 0.06 | ||
| FSI | Osteopenic | 50 | 1.46 | 0.13 | 0.05* |
| Osteoporotic | 25 | 1.13 | 0.27 | ||
| CSMI | Osteopenic | 50 | 6.98 | 1.56 | 0.01* |
| Osteoporotic | 25 | 5.94 | 1.17 | ||
| CSA | Osteopenic | 50 | 117.67 | 12.54 | 0.000* |
| Osteoporotic | 25 | 98.03 | 14.29 | ||
| HAL | Osteopenic | 50 | 35.59 | 7.87 | 0.44 |
| Osteoporotic | 25 | 34.23 | 11.21 | ||
| NSA | Osteopenic | 50 | 126.1 | 1.19 | 0.05* |
| Osteoporotic | 25 | 127.9 | 1.36 |
p<0.05 represents statistically significant results (*)
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Leichter I, Margulies JY, Weinreb A, et al. The relationship between bone density, mineral content and mechanical strength in the femoral neck. Clin Orthop. 1982;163:272–81. [PubMed] [Google Scholar]
- 2.Dalen N, Hellstrom LG, Jacobson B. Bone mineral content and mechanical strength in the femoral neck. Acta Orthop Scand. 1976;47:503–8. doi: 10.3109/17453677608988728. [DOI] [PubMed] [Google Scholar]
- 3.Faulkner KG, Wacker WK, Barden HS, et al. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int. 2006;17:593–9. doi: 10.1007/s00198-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–7. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, III, Wahner HW, Richelson LA, O’Fallon WM, Riggs B. Osteoporosis and the risk of hip fracture. Am J Epidemiol. 1986;124:254–61. doi: 10.1093/oxfordjournals.aje.a114383. [DOI] [PubMed] [Google Scholar]
- 6.Ross PD, Davis JB, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–23. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Wahner HW, Seeman KP, Offord, et al. Changes in bone mineral density of the proximal femur and spine with aging. J Clin Inves. 1982;70:716–23. doi: 10.1172/JCI110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohr H, Schaadt O. Bone femoral content of femoral bone and lumbar spine measured in women with fracture of the femoral neck by dual photon absorptiometry. Clin Orthop. 1983;179:240–5. [PubMed] [Google Scholar]
- 9.Norimatsu H, Mori S, Uesato T, Yoshikawa T, Katsuyama N. Bone mineral density of the spine and proximal femur in normal and osteoporotic subjects in Japan. Bone Miner. 1989;5:213–22. doi: 10.1016/0169-6009(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 10.Tabensky A, Duan Y, Edmonds J, Seman E. The contribution of reduced peak accrual of bone and age-related bone loss to osteoporosis at the spine and hip: insights from the daughters of women with vertebral or hip fractures. J Bone Miner Res. 2001;16:1101–7. doi: 10.1359/jbmr.2001.16.6.1101. [DOI] [PubMed] [Google Scholar]
- 11.Rivadeneira F, Zillikens MC, De Laet CE, et al. Femoral Neck BMD is a Strong Predictor of Hip Fracture Susceptibility in Elderly Men and Women Because it Detects Cortical Bone Instability: The Rotterdam Study. J Bone Miner Res. 2007;22:1781–90. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 12.Peacock M, Turner CH, Liu G, Manatunga AK, Timmenman L, Johnston CC., Jr Better discrimination of hip fracture using bone density, geometry and architecture. Osteoporos Int. 1995;5:167–73. doi: 10.1007/BF02106096. [DOI] [PubMed] [Google Scholar]
- 13.Bonen S, Koutri R, Dequeker J, et al. Measurement of femoral geometry in type I and type II osteoporosis: Differences in hip axis length consistent with heterogeneity in the pathogenesis of osteoporotic fractures. J Bone Miner Res. 1995;10:1908–12. doi: 10.1002/jbmr.5650101210. [DOI] [PubMed] [Google Scholar]
- 14.Lewiecki EM. Managing osteoporosis: Challenges and strategies. Cleveland Clinic Journal of medicine. 2009;76:457–66. doi: 10.3949/ccjm.76a.09019. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, Carbone L, Cheng H, et al. Longitudinal patterns in bone mass measurement among U.S. Medicare beneficiaries. J Bone Miner Ress. 2007;22:5193. [Google Scholar]
- 16.Kanis JA, on behalf of the World Health Organization Scientific Group . Assessment of osteoporosis at the primary health-care level. 2008. Technical Report. World Health Organization Collaborating Centre for Methabolic Bone Diseases, University of Sheffield, UK. [Google Scholar]
- 17.Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90:2787–93. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- 18.Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–12. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa T, Turner CH, Peacock M, et al. Geometric structure of the femoral neck measured using Dual-Energy X-ray Absorptiometry. J Bone Miner Res. 1994;9:1053–64. doi: 10.1002/jbmr.5650090713. [DOI] [PubMed] [Google Scholar]
- 20.Melton JL, Beck EJ, Amin S, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos Int. 2005;16:460–7. doi: 10.1007/s00198-004-1820-1. [DOI] [PubMed] [Google Scholar]
- 21.Duan Y, Beck TJ, Wang XF, Seman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res. 2003;18:1766–74. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 22.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–50. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 23.Mayhew PM, Thomas CD, Clement JG, et al. Relation between age, femoral neck cortical stability and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 24.Ruff CB. Body size, body shape, and long bone strength in modern humans. J Hum Evol. 2000;38:269–90. doi: 10.1006/jhev.1999.0322. [DOI] [PubMed] [Google Scholar]
- 25.Boudoin C, Fardellone P, Sebert JL. Effect of sex and age on the rate of cervical to trochanteric hip fracture. A meta-analysis of 16 reports on 36.451 cases. Acta Orthop Scand. 1993;64:647–53. doi: 10.3109/17453679308994590. [DOI] [PubMed] [Google Scholar]
- 26.Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. Hip structural geometry in old and old age: Similarities and differences between men and women. Bone. 2007;41:722–32. doi: 10.1016/j.bone.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnudi S, Ripamonti C, Gualtieri G, Malavolta N. Geometry of proximal femur in the prediction of hip fracture in osteoporotic women. Br J Radiol. 1999;72860:729–33. doi: 10.1259/bjr.72.860.10624337. [DOI] [PubMed] [Google Scholar]
- 28.Alonso CG, Diaz MD, Carrenza FH, et al. The Multicenter Project for Research in Osteoporosis femoral bone mineral density, neck-sshaft angle and mean femoral neck width as predictors of hip fracture in men and women. Osteoporos Int. 2000;11:714–20. [PubMed] [Google Scholar]


