Abstract
Objective:
Although there are limited numerous reports of candidemia in adults, data on paediatrics are stil limeted. The aim of the present study was to compare the aetiology and risk factors of nosocomial candidemia among the paediatric and adults in our hospital.
Materials and Methods:
This study includes the patients hospitalised and diagnosed as fungemia at Ondokuz Mayis University Hospital between June 30, 2007 and June 30, 2009 whose blood cultures sent to our microbiology laboratory. After fungal growth was observed in blood cultures, the yeast cells were inoculated onto Saboraud glucose agar. The colonies were identified by conventional yeast identification methods and ID 32C yeast identification system according to the manifacturer’s instructions.
Results:
During this period 51 paediatric and 69 adults were studied. The most common yeast form was Candida albicans (43.3%) followed by C. parapsilosis (25.0%) and C. tropicalis (17.5%). Although the non-albicans Candida species represent more than half (56.7%) of all candidemic cases C. albicans was the most common frequent etiologic agent. There was no statistically significant difference between patient age (paediatric and adult) and distribution of Candida species (p>0.05) Neoplasia (in adults) and prematurity (in paediatrics) were the main underlying diseases. Predisposing factors and mortality rates were not different among paediatrics and adults.
Conclusion:
We reinforce the necessity of continous epidomiologic surveillance to follow the dynamics of candidemia.
Keywords: Candida, Bloodstream infection, Epidemiology, Mortality, Risk factors
Özet
Amaç:
Yetişkinlerde kandidemi ile ilgili çok fazla bildiri olmasına rağmen çocuklarla ilgili bilgiler hala sınırlıdır. Bu çalışmanın amacı, hastanedeki çocuk ve yetişkinlerdeki nozokomiyal kandidemi etiyoloji ve risk faktörlerinin karşılaştırmaktır.
Gereç ve Yöntem:
Bu çalışma, 30 Haziran 2007’ile 30 Haziran 2009 arasında kan kültürleri mikrobiyoloji laboratuarına gönderilmiş olan hastanede yatan ve fungemi tanısı almış olanları kapsamaktadır. Kan kültüründe maya üremesi gözlendikten sonra maya hücreleri Saboraud glikoz agara inoküle edilmişlerdir. Koloniler konvansiyonel maya tanımlama yöntemleri ve üretici firmanın önerileri doğrultusunda ID 32C maya tanımlama sistemi ile tanımlanmışlardır.
Bulgular:
Bu süre zarfında 51 çocuk ve 69 yetişkin hasta çalışılmıştır. En çok görülen maya Candida albicans (%43.3) olup bunu C. parapsilosis (%25) ve C. tropicalis (%17.5) izlemiştir. Non-albicans Candida türleri bütün kandidemik vakaları yarısından fazlasını oluşturmasına rağmen (%56.7) C. albicans en yaygın olan etiyolojik ajandır. İstatistiksel olarak hasta yaşı (çocuk ve yetişkin) ve Candida (p>0.05) türleri arasında anlamlı bir fark bulunmamıştır. Neoplazi (yetişkinlerde) ve prematürite (çocuklarda) altta yatan an hastalıklardandır. Predispozan faktörler ve mortalite oranları, çocuk ve yetişkinlerde farklılık göstermemektedir.
Sonuç:
Kandidemi değişimlerini takip edebilmek için sürekli epidemiyolojik sürveyansın gerekliliğine inanmaktayız.
Introduction
The incidence of the nosocomial fungal infections has increased over the past two decades. Candida spp. have become the fourth most common cause of nosocomial bloodstream infections [1–3]. During this period, the isolation rate of non-albicans Candida spp. has increased as well. The species distribution of Candida isolates varies between countries, regions and institutions [1, 4]. Several studies have determined the risk factors fungal infections. These risk factors include the length of stay in the intensive care unit, the use of intravascular catheters, malignancy, surgical operations, chemotherapy, antimicrobial agents and steroid use [2, 5–9].
Although there have been numerous studies of candidemia in adults [2, 5–8] data on pediatric populations [9–12] are still limited. The aim of this study was to compare the etiology and risk factors of nosocomial candidemia among pediatric and adult patients at a university hospital in 2007–2009.
Materials and Methods
Study population
A retrospective study was performed between June 30, 2007 and June 30, 2009 in the 900-bed Ondokuz Mayis University Hospital. All pediatric and adult cases of nosocomial candidemia were included in this study. Nosocomial candidemia was defined as the presence of at least one blood culture positive for Candida obtained from a peripheral vein in a patient admitted for more than 72 h in association with temporally related signs and symptoms. Patients were considered pediatric if their age was less than 18 years old.
The following variables were studied: age, sex, and underlying diseases. The predisposing factors that we reviewed in the patients’ data included the following: antimicrobial chemotherapy, parenteral nutrition, vascular catheterization, urinary catheters, endotracheal intubation, hemodialysis, tracheostomy, surgery, prolonged intensive care unit stay, and steroid therapy.
Microbiological procedures
Blood cultures were processed at a microbiology laboratory using an automated blood culture system (BacT/ALERT® 3D, bioMérieux, France). Passages on Sabouraud dextrose agar (SDA) (Merck, Darmstadt, Germany) were performed. After screening using the germ-tube test, yeast were identified according to their morphology on cornmeal Tween 80 agar, colony morphology and color on CHROMagar Candida (BBL, Becton Dickinson, Sparks, MD, USA) and biochemical tests using an API ID 32C yeast identification system (bioMérieux, Marcy l’Etoile, France). Only one isolate from each patient was included. Three reference strains (Candida albicans ATCC 26255, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019) were included in this study.
Statistical analysis
Descriptive statistics were used to summarize the data. Pearson’s chi-square test and Fisher exact test were used to evaluate the association between qualitative variables, and the Mann-Whitney test was used for the comparison of quantitative variables. The two-tailed level of significance was 5%, and data analysis was performed with SPSS software. Survival rates were evaluated by Kaplan-Meier analysis and the long rank test.
Results
During the period of study, 120 patients had nosocomial candidemia in our hospital. There were 51 (42.5%) pediatric patients and 69 (57.5%) adult patients. Most of our pediatric patients were male (51.0%), and the median age was 1.0 year old (mean 3.86±4.737). Most of the adults with nosocomial candidemia were male (56.5%), and the median age was 54.0 years old (range, 19–83 years old).
Although non-albicans Candida species represented more than half (56.7%) of all candidemia cases, C. albicans was the most frequent etiological agent (43.3%) of candidemia in our hospital. The most common non-albicans species isolated was C. parapsilosis (25.0%), followed by C. tropicalis (17.5%). Fluconazole-resistant species, such as C. glabrata (5.0%) and C. krusei (3.3%), were isolated rarely in our patients. Table 1 shows the distribution of Candida species among the pediatric and adult patients with nosocomial candidemia. There was no statistically significant difference in the distribution of Candida species between pediatric and adult patients (p>0.05), but C. dubliniensis, C. guilliermondii and C. lusitaniae were isolated only in pediatric patients in our study. In addition, C. kefyr was isolated only from adults.
Table 1.
Candida species distribution among children and adults with nosocomial candidemia
| Candida species | Children (n=51) | Adults (n=69) | Total (n=120) |
|---|---|---|---|
| C. albicans | 39.2% | 46.4% | 43.3% |
| Non-Candida albicans species | 60.8% | 53.6% | 56.7% |
| C. parapsilosis | 23.5% | 26.1% | 25.0% |
| C. tropicalis | 17.6% | 17.4% | 17.5% |
| C. glabrata | 3.9% | 5.8% | 5.0% |
| C. krusei | 5.9% | 1.4% | 3.3% |
| C. guilliermondii | 5.9% | 0.0% | 2.5% |
| C. kefyr | 0.0% | 2.9% | 1.7% |
| C. dubliniensis | 2.0% | 0.0% | 0.8% |
| C. lusitaniae | 2.0% | 0.0% | 0.8% |
There was no statistically significant difference in the distribution of Candida species between pediatric and adults patients (p>0.05)
Table 2 shows the major underlying diseases in pediatric and adult patients with nosocomial candidemia. The major underlying diseases observed in the pediatric patients were prematurity (25.5%), neoplasia (17.6%), and infection (15.7%). The major underlying diseases observed in the adults were neoplasia (36.2%), trauma (13.0%), and infection (17.4%).
Table 2.
Underlying diseases among children and adults with nosocomial candidemia
| Underlying diseases | Children (n=51) | Adults (n=69) | Total (n=120) |
|---|---|---|---|
| Malignancy | 17.6% | 36.2% | 28.3% |
| Infection | 15.7% | 17.4% | 16.7% |
| Vascular disease | 11.8% | 11.6% | 11.7% |
| Prematurity | 25.5% | 0.0% | 10.8% |
| Trauma | 7.8% | 13.0% | 10.8% |
| Hereditary syndromes | 11.8% | 1.5% | 5.8% |
| Renal failure | 0.0% | 8.7% | 5.0% |
| Others | 9.8% | 11.6% | 10.8% |
Table 3 shows the predisposing factors for the pediatric and adult cases with nosocomial candidemia, including the following: antimicrobial chemotherapy, parenteral nutrition, vascular catheterization, urinary catheter, endotracheal intubation, hemodialysis, tracheotomy, surgery, prolonged intensive care unit stay, and steroid therapy. There was no statistically significant difference in the predisposing factors between pediatric and adult patients with nosocomial candidemia (p>0.05). We also evaluated the relationship between the presence of predisposing factors and the etiological agent that was isolated. There was no statistically significant difference in the predisposing factors present between infections caused by C. albicans and those caused by non-albicans species.
Table 3.
Paediatric versus adult nosocomial candidemia: Predisposing factors
| Predisposing factors | Paediatrics (%) | Adults (%) |
|---|---|---|
| Antimicrobial chemotherapy | 76.4 | 73.9 |
| Parenteral nutrition | 72.5 | 62.3 |
| Vascular catheterization | 39.2 | 36.2 |
| Urinary catheter | 23.5 | 34.7 |
| Endotracheal entubation | 17.6 | 20.2 |
| Surgery | 15.6 | 11.5 |
| Hemodyalise | 0 | 7.2 |
| Tracheotomy | 3.9 | 2.8 |
| Prolonged intensive care unit stay | 1.9 | 2.8 |
| Steroid therapy | 0 | 1.4 |
There was no statistically significant difference in predisposing factors between paediatric and adult with nosocomial candidemia (p>0.05)
When time-to-death estimates were evaluated with the Kaplan-Meier method and compared using the log-rank technique (Figure 1), no statistically significant difference between pediatric and adult patients with nosocomial candidemia was observed.
Figure 1.
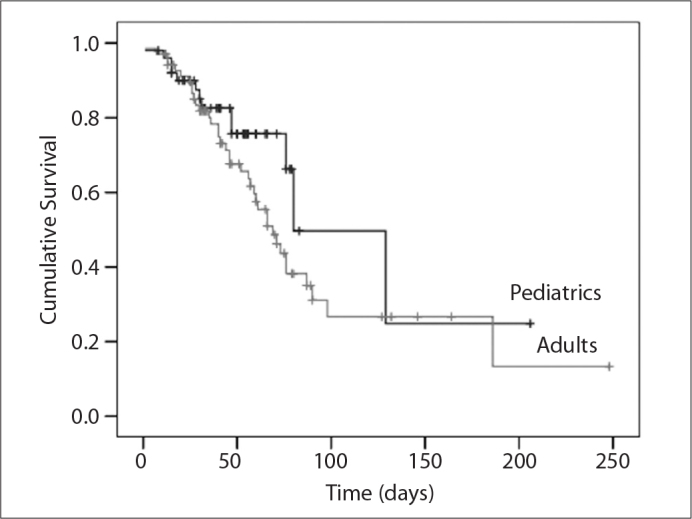
Time-to-death estimates for the pediatric and adult groups using the Kaplan- Meier method.
Discussion
Although non-albicans Candida species represented more than half of all candidemia cases, the present study determined that C. albicans is the most frequent etiological agent of candidemia in our hospital. This finding, together with data from other countries, confirms that C. albicans is still a leading cause of candidemia [1, 3, 6–8, 10, 11]. Nevertheless, the prevalence of C. albicans (43.3%) in this study was lower than that reported in Canada (59%), Europe (58%), and the USA (54%); however, this prevalence was similar to the prevalences in Korea (49%), Latin America (47%), Israel (44%), and Japan (41%) [1, 13]. In this study, C. parapsilosis was the most common non-albicans species identified, which is consistent with data from Japan, Canada, South America, and Korea [14]. Fluconazole-resistant species, such as C. glabrata and C. krusei, were rarely isolated from our patients. The comparison of Candida spp. between pediatric and adult patients revealed no significant difference, but C. dubliniensis, C. guilliermondii and C. lusitaniae were isolated only in pediatric patients in our study. In addition, C. kefyr was isolated only from adult patients. In some studies, C. parapsilosis had been found to be the main etiological agent in pediatric patients [9], but in our study, C. parapsilosis was the second most common etiological agent after C. albicans. In addition, C. parapsilosis has been found to be associated with the use of central venous catheters and total parenteral nutrition [5]. We did not find any difference in the prevalences of C. albicans and C. parapsilosis among risk factors such as the use of central venous catheters and total parenteral nutrition. Neoplasia was the main underlying disease in adults. The use of steroids and immunosuppressors in patients with neoplasia may be risk factors for candidemia. Prematurity was the most frequent underlying disease in pediatric patients; the reason for candidemia in premature infants may be the immaturity of the immune system in neonates and the use of broad-spectrum antibiotics to treat infections. Most of our patients had received broad-spectrum antimicrobials and total parenteral nutrition.
In our study, the mortality rates were not different between the pediatric and adult patients. This finding differed from those of previous studies. Numerous studies [10, 11, 15, 16] have found lower mortality rates in pediatric patients than in adults. In a large, prospective study of candidemia, it was shown that the mortality rate due to candidemia caused by C. parapsilosis was usually lower than that of candidemia caused by other Candida species. This difference may play a role in the reported mortality differences [11]. In our study, the distribution of C. parapsilosis was not different between adults and children.
In conclusion, we reviewed data on the species distribution, underlying diseases and predisposing factors related to cases of nosocomial candidemia among pediatric and adult patients over a two-year period at a tertiary university hospital in Turkey. These data suggest that C. albicans is the most frequent etiological agent of candidemia in our hospital, although non-albicans Candida species represented more than half of all candidemia cases. The most common non-albicans species was C. parapsilosis in both adults and children in our hospital. There was no statistically significant difference in the distribution of Candida species between pediatric and adult patients. Neoplasia (in adults) and prematurity (in children) were the main underlying diseases. The predisposing factors and mortality rates were not different between the pediatric and adult patients. Our study was limited because the data were derived from a single institution, the design was retrospective, and there was no control group without candidemia. Further studies are needed to fully analyze the dynamics of candidemia.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Samra Z, Yardeni M, Peled N, Bishara J. Species distribution and antifungal susceptibility of Candida bloodstream isolates in a tertiary medical center in Israel. Eur J Clin Microb Infect Dis. 2005;24:592–5. doi: 10.1007/s10096-005-0005-y. [DOI] [PubMed] [Google Scholar]
- 2.Eryüksel E, Ergönül O, Olgun S, Odabasi Z, Korten V, Celikel T. Risk factors for mortality in fungal infections. Turkiye Klinikleri J Med Sci. 2009;29:99–103. [Google Scholar]
- 3.Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot JL, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–74. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 4.2002. National Committee for Clinical Laboratory Standards: Reference method for broth dilution antifungal susceptibility testing of yeasts; approved Standard. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards. Wayne, PA.
- 5.Yapar N, Uysal U, Yucesoy M, Cakir N, Yuce A. Nosocomial bloodstream infections associated with Candida species in a Turkish University hospital. Mycoses. 2006;49:134–8. doi: 10.1111/j.1439-0507.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Pasqualotto AC, Nedel WL, Machado TS, Severo LC. Risk factors and outcome for nosocomial breakthrough candidaemia. J Infect. 2006;52:216–22. doi: 10.1016/j.jinf.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Bouza E, Munoz P. Epidemiology of candidemia in intensive care units. International Journal of Antimicrobial Agents. 2008;32:87–91. doi: 10.1016/S0924-8579(08)70006-2. [DOI] [PubMed] [Google Scholar]
- 8.Boo TW, O’Reilly B, O’Leary J, Cryan B. Candidaemia in an Irish tertiary referral hospital: epidemiology and prognostic factors. Mycoses. 2005;48:251–9. doi: 10.1111/j.1439-0507.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto AC, Nedel WL, Machado TS, Severo LC. A 9-year study comparing risk factors and the outcome of paediatrics and adults with nosocomial candidaemia. Mycopathol. 2005;160:111–6. doi: 10.1007/s11046-005-3452-1. [DOI] [PubMed] [Google Scholar]
- 10.Moran C, Grussemeyer CA, Spalding JR, Benjamin DK, Reed SD. Candida albicans and non-albicans bloobstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr Infect Dis J. 2009;28:433–5. doi: 10.1097/INF.0b013e3181920ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG, Rex JH, Lee J, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clinical Infectious Diseases. 2003;37:634–43. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 12.Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635–41. doi: 10.1097/01.inf.0000128781.77600.6f. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Diekma DJ, for the International Fungal Surveillance Participant Group Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates. Clin Microbiol Infect. 2004;10:11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Shin JH, Lee K, et al. Species distribution and susceptibility to azole antifungals of Candida bloodstream isolates from eight university hospitals in Korea. Yonsei Med J. 2007;48:779–86. doi: 10.3349/ymj.2007.48.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco-Rios A, Avila-Figueroa C, Nobigrot-Kleinman D, Santos JI. Mortality associated with systemic candidiasis in children. Arch Med Res. 1997;28:229–32. [PubMed] [Google Scholar]
- 16.Stamos JK, Rowley AH. Candidemia in a pediatric population. Clin Infect Dis. 1995;20:571–5. doi: 10.1093/clinids/20.3.571. [DOI] [PubMed] [Google Scholar]


