Abstract
Objective:
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne viral hemorrhagic fever. Disseminated intravascular coagulation (DIC) is an important complication of this disease, especially in severe and fatal cases. Antithrombin (AT) acts as an anticoagulant by inactivating thrombin, Factor IX, Factor X and Factor XI. We conducted this study to investigate the AT levels and their prognostic value in CCHF.
Materials and Methods:
Twenty-eight confirmed CCHF patients were included in this study. Diagnosis of the disease was made by CCHF IgM and/or PCR positivity. Patients were grouped based on the severity criteria described previously. The patients with platelet counts <20 000×106 cell/L, white blood counts >10×109 cell/L, prothrombin times >60 seconds, aspartate aminotransferase levels >700 IU/L or alanine aminotransferase levels >900 IU/L were accepted as severe cases. Patients whose illnesses were self-limited and who did not require blood component replacement were accepted as mild cases, and patients who improved but required blood component replacement were accepted as moderate cases. Blood samples were obtained on the day that the patient had the lowest platelet count and before any thrombocyte replacement. The antithrombin activity was measured using a chromogenic substrate test (Diagnostica Stago STA Compact) at a research laboratory.
Results:
Twenty-two (78.6%) of the cases were mild, 3 (10.7%) were moderate, and 3 were (10.7%) severe. The mean AT value was 101% for mild cases, 116.6 % for moderate cases, and 88% for severe cases (p>0.05). Although there were no statistically significant differences between the AT values, the mean AT activity was lower in severe CCHF cases.
Conclusion:
The AT activity may have been decreased in severe CCHF cases. Further studies with greater numbers of patients are required to determine the level of AT activity and its correlation with disease severity and the prognosis of CCHF.
Keywords: Antithrombin, Coagulopathy, Crimean-Congo Hemorrhagic Fever
Özet
Amaç:
Kırım-Kongo Kanamalı Ateşi (KKKA) kene kaynaklı bir viral kanamalı ateştir. Yaygın damariçi pıhtılaşma (YDP) KKKA’da özellikle ciddi ve ölümcül olgularda görülen önemli bir komplikasyondur. Antithrombin (AT) antikoagülan bir proteindir ve temelde trombin, Faktör IX, Faktör X ve Faktör XI’i inaktive eder. Bu çalışma KKKA’da AT aktivitesini ve prognostik değerini araştırmak amacıyla yürütüldü.
Gereç ve Yöntem:
Çalışmaya kesin KKKA tanılı 38 hasta dahil edildi. KKKA tanısı KKKA IgM ve/veya PCR pozitifliği ile konuldu. Hastalar daha önce tanımlanmış ciddiyet kriterlerine göre sınıflandırıldı. Trombosit sayısı <20 000×106 cell/L veya beyaz küre sayısı >10000×106 cell/L veya protrombin zamanı >60 saniye veya aspartat aminotransferaz düzeyi >700 IU/L ya da alanin aminotransferaz >900 IU/L olan hastalar ciddi olgu olarak kabul edildi. Ek olarak kendiliğinden düzelen, herhangi bir kan ürünü ihtiyacı gerektirmeyen hastalar hafif, iyileşen ancak replasman gerektiren hastalar orta ciddiyette kabul edildi. Hastalardan trombosit değerinin en düşük olduğu gün, trombosit replasmanı yapılmadan önce kan örneği alındı. AT aktivitesi kromojenik substrat testi ile rutin araştırma laboratuarında ölçüldü (Diagnostica Stago STA Compact).
Bulgular:
Çalışmaya alınan hastaların 32’si (%78.6) hafif, 3’ü (%10.7) orta, 3’ü (%10.7) ağır hasta idi. Ortalama AT düzeyleri hafif olgularda %101, orta olgularda %116.6 ve ağır olgularda %88 idi (p>0.05). İstatistiksel anlama ulaşmasa da ortalama AT seviyesi ciddi olgularda göreceli olarak düşüktü.
Sonuç:
KKKA’da AT aktivitesi ciddi olgularda azalabilmektedir. AT aktivitesinin hastalığın ciddiyeti ve prognozu ile ilişkisinin saptanması için daha fazla sayıda hasta ile yapılan ileri çalışmalara ihtiyaç vardır.
Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne viral hemorrhagic fever. In the last decade, this disease has become endemic in Turkey and its neighbors [1]. Bleeding due to thrombocytopenia and/or disseminated intravascular coagulation (DIC) is an important complication of CCHF. DIC is seen in severe and fatal cases. The disease pathogenesis is not clear. However, it is known that the major targets of the disease are endothelial cells, hepatocytes and mononuclear phagocytes [2]. Endothelial damage, vasculitis and sepsis occur in patients with CCHF [3].
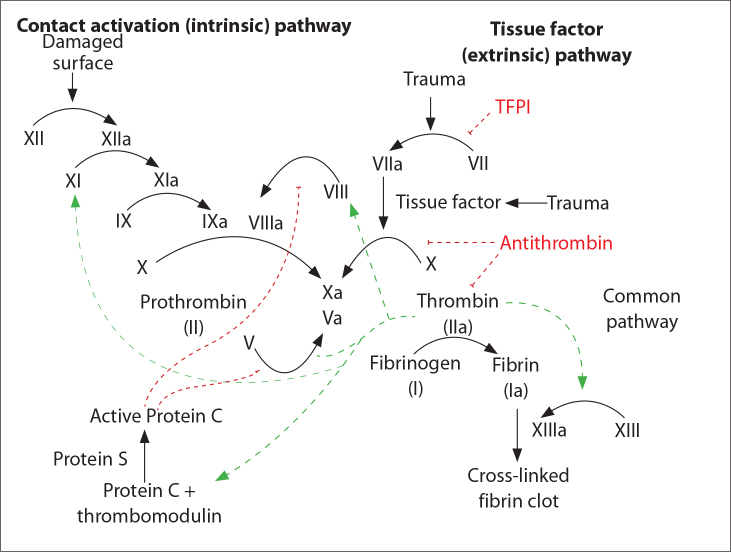
Antithrombin (AT) is a 58-kDa serine protease inhibitor (serpin) that acts as an anticoagulant protein by inactivating thrombin, Factor IX, Factor X and Factor XI (Figure 1) [4]. AT is synthesized in the liver, and plasma levels tend to be low in individuals with various pathological conditions such as liver cirrhosis, DIC, nephrotic syndrome and acute thrombosis [4]. AT had been successfully used as a supportive therapy in experimental endotoxin-induced DIC models [5].
Figure 1.
Blood coagulation pathways.
We conducted this study to investigate the AT levels and their prognostic significance in CCHF.
Materials and Methods
Patients
This study was conducted at a tertiary hospital in 2007, and 38 confirmed CCHF patients were enrolled. Diagnosis of the disease was made by CCHF IgM and/or PCR positivity. Patients were grouped based on the severity criteria described previously [6]. The patients with platelet counts ≤20 000×106 cell/L, white blood counts ≥10000×106 cell/L, prothrombin times ≥ 60 seconds, aspartate aminotransferase levels ≥700 IU/L or alanine aminotransferase levels ≥900 IU/L were accepted as severe cases. Patients whose illnesses were self-limited and who did not require blood component replacement were accepted as mild cases, and patients who improved but required blood component replacement were accepted as moderate cases.
Test Procedure
Venous blood samples were collected in tubes containing EDTA, and AT activity was detected the same day. Antithrombin activity was measured using a chromogenic substrate test (Diagnostica Stago STA Compact) at a routine research laboratory. The normal range was considered to be 70–110%.
Statistical analysis
The data were analyzed using SPSS 11.5 (SPSS Inc., Chicago, IL). The Mann-Whitney U test was used to compare the parameters, and p values lower than 0.05 were accepted as significant.
Results
Twenty-two (78.6%) of the cases were mild, 3 (10.7%) were moderate, and 3 were (10.7%) severe. The mean AT values were 101% for mild cases, 116.6% for moderate cases, and 88% for severe cases (p>0.05) (Figure 2). Although the mean values for the antithrombin activity were all in the normal range and the differences between these values were not statistically significant due to the small number of the cases, the mean AT activity was lower for severe CCHF cases.
Figure 2.
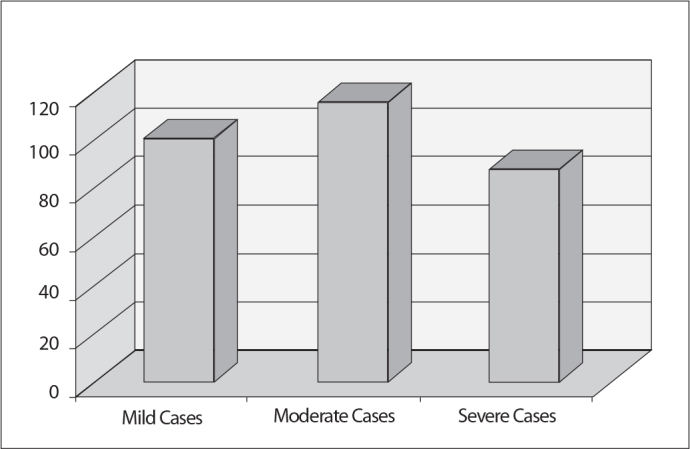
Mean AT activity in CCHF patients.
Discussion
Under homeostatic conditions, the body is maintained in a finely tuned balance between coagulation and fibrinolysis. DIC involves the dysregulation of the processes of coagulation and fibrinolysis, and the result is widespread clotting with resultant bleeding. Viral infections may perturb hemostasis and can lead to thrombohemorrhagic complications or syndromes such as DIC or vasculitis [7]. Endothelial cell injury is a common feature of some viral infections and can alter hemostasis in a direct or indirect manner. Endothelial damage contributes to hemostatic failure as the result of the consequent activation of the intrinsic coagulation cascade [7]. The ability to infect endothelial cells has been demonstrated for viruses that cause hemorrhagic fevers, such as the dengue, Marburg, Ebola, Hantaan, and Lassa viruses [8].
The pathogenesis of CCHF is still unclear. Changes in the levels of coagulation factors and anticoagulant proteins and alterations to the fibrinolytic system in patients with CCHF have been investigated in a limited number of studies [9–11].
Sonmez et al. found low thrombin-activatable fibrinolysis inhibitor (TAFI) activity in CCHF patients; TAFI is an important protein affecting the stability of fibrin clots. The researchers concluded that low levels of TAFI activity may be an important factor leading to an imbalance in fibrinolysis, resulting in bleeding complications in patients with CCHF [9].
We kinetically investigated d-dimer, protein C, protein S, and Factor VIII in CCHF patients in a previous study. We found that d-dimer has a prognostic value in the disease and that protein C and protein S activity may decrease during the acute phase. These activity levels returned to normal in patients who survive but were persistently low in fatal cases [10]. Therefore, we suggest that the coagulation and fibrinolysis balance is affected during CCHF, as in other viral hemorrhagic fevers.
Onguru et al. investigated the same coagulation and fibrinolysis markers in CCHF. They found that there were no statistically significant differences with respect to the AT values and other parameters (protein C, protein S activity, d-dimer) between fatal and nonfatal cases [11].
There have been many studies investigating AT activity in other viral hemorrhagic diseases. The AT levels were found to be in the normal range in individuals with Argentine hemorrhagic fever and hemorrhagic fever with renal syndrome [12, 13].
It was found that AT may transiently decrease during Korean fever and dengue hemorrhagic fever in the acute stage but returns to normal during the recovery period [14, 15].
AT levels have also been found to be decreased in patients with dengue shock syndrome, hemorrhagic fever with renal syndrome and epidemic hemorrhagic fever [16–20]. In an experimental study, low levels were observed in guinea pigs [21].
In this study, we found no difference in the AT values between mild, moderate, and severe cases of CCHF. Although there was no statistically significant difference, the AT activity was lower in severe cases. The number of severe cases was low, and there were no fatal cases in our series. Therefore, the statistical analysis may have been affected by the small number of severe cases.
In conclusion, to understand AT changes, further studies are need with more patients. In addition, the changes in AT should be monitored daily.
Acknowledgments
We thank the Refik Saydam Hygiene Center for the micro-biological diagnosis of the disease.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Leblebicioglu H. Crimean-Congo haemorrhagic fever in Eurasia. Int J Antimicrob Agents. 2010;36:43–6. doi: 10.1016/j.ijantimicag.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Burt FJ, Swanepoel R, Shieh WJ, et al. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med. 1997;121:839–46. [PubMed] [Google Scholar]
- 3.Bodur H, Akinci E, Ongürü P, Uyar Y, Baştürk B, Gözel MG. Evidence of vascular endothelial damage in Crimean-Congo hemorrhagic fever. Int J Infect Dis. 2010;14:704–7. doi: 10.1016/j.ijid.2010.02.2240. [DOI] [PubMed] [Google Scholar]
- 4.Khor B, Van Cott EM. Laboratory tests for antithrombin deficiency. Am J Hematol. 2010;85:947–50. doi: 10.1002/ajh.21893. [DOI] [PubMed] [Google Scholar]
- 5.Davis-Jackson R, Correa H, Horswell R, et al. Antithrombin III (AT) and recombinant tissue plasminogen activator (R-TPA) used singly and in combination versus supportive care for treatment of endotoxin-induced disseminated intravascular coagulation (DIC) in the neonatal pig. Thromb J. 2006;18:4–7. doi: 10.1186/1477-9560-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ergonul O, Celikbas A, Baykam N, Eren S, Dokuzoguz B. Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: severity criteria revisited Clin Microbiol Infect. 2006;12:551–4. doi: 10.1111/j.1469-0691.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Gorp EC, Suharti C, ten Cate H, et al. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180:176–86. doi: 10.1086/314829. [DOI] [PubMed] [Google Scholar]
- 8.Schnittler HJ, Feldmann H. Viral hemorrhagic fever-a vascular disease? Thromb Haemost. 2003;89:967–72. [PubMed] [Google Scholar]
- 9.Sonmez M, Aydin K, Durmus A, et al. Plasma activity of thrombin activatable fibrinolysis inhibitor in Crimean-Congo hemorrhagic fever. J Infec. 2007;55:184–7. doi: 10.1016/j.jinf.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Ozkurt Z, Erol S, Kadanali A, Ozden K. Protein C, Protein S levels and other hematological parameters in patients with Crimean congo hemorrhagic fever. Preceedings of 19th European Congress of Clinical Microbiology and Infectious Diseaeses; Helsinki, Finland. 16–19 May 2009; p. 581. [Google Scholar]
- 11.Onguru P, Dagdas S, Bodur H, et al. Coagulopathy parameters in patients with Crimean-Congo hemorrhagic fever and its relation with mortality. J Clin Lab Anal. 2010;24:163–6. doi: 10.1002/jcla.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller MV, Marta RF, Sturk A, et al. Early markers of blood coagulation and fibrinolysis activation in Argentine hemorrhagic fever. Thromb Haemost. 1995;73:368–73. [PubMed] [Google Scholar]
- 13.Davidovich IM, Parshina TA, Utkin VN. Von Willebrand factor, antithrombin III and 5’-nucleotidase in the blood and the athrombogenic properties of the vascular wall in patients with hemorrhagic fever with renal syndrome. Ter Arkh. 1993;65:22–5. [PubMed] [Google Scholar]
- 14.Lee M. Coagulopathy in patients with hemorrhagic fever with renal syndrome. J Korean Med Sci. 1987;2:201–11. doi: 10.3346/jkms.1987.2.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funahara Y, Sumarmo, Wirawan R. Features of DIC in dengue hemorrhagic fever. Bibl Haematol. 1983;49:201–11. doi: 10.1159/000408460. [DOI] [PubMed] [Google Scholar]
- 16.Wills BA, Oragui EE, Stephens AC, et al. Coagulation abnormalities in dengue hemorrhagic Fever: serial investigations in 167 Vietnamese children with Dengue shock syndrome. Clin Infect Dis. 2002;35:277–85. doi: 10.1086/341410. [DOI] [PubMed] [Google Scholar]
- 17.Funahara Y, Sumarmo, Shirahata A, Setiabudy-Dharma R. DHF characterized by acute type DIC with increased vascular permeability. Southeast Asian J Trop Med Public Health. 1987;18:346–50. [PubMed] [Google Scholar]
- 18.Wei L, Pan X, Yu Y, Lu Q. The changes and relation among platelet function, plasma heparin and anti-coagulation-III: activity in patients with hemorrhagic fever with renal syndrome. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1997;11:163–5. [PubMed] [Google Scholar]
- 19.Zhang TM, Yang ZQ, Zhang MY, et al. Early analysis of viremia and clinical tests in patients with epidemic hemorrhagic fever. Chin Med J. 1993;106:608–10. [PubMed] [Google Scholar]
- 20.Xiang LB, Zhang ZY, Chen JM, Xiang JM, Cosgriff TM. Epidemic hemorrhagic fever. The mechanism of coagulation and fibrinolysis. Chin Med J. 1990;103:391–5. [PubMed] [Google Scholar]
- 21.Cosgriff TM, Jahrling PB, Chen JP, et al. Studies of the coagulation system in arenaviral hemorrhagic fever: experimental infection of strain 13 guinea pigs with Pichinde virus. Am J Trop Med Hyg. 1987;36:416–23. doi: 10.4269/ajtmh.1987.36.416. [DOI] [PubMed] [Google Scholar]