Abstract
Objective:
Midazolam and dexmedetomidine, which are used for sedation during endoscopic retrograde cholangiopancreatography, were compared to evaluate the differences in efficacy, hemodynamics, and side effects.
Materials and Methods:
Fifty patients aged between 18 and 80 were randomly assigned to two groups according to American Society of Anesthesiologists (ASA) classification: Group M received midazolam with an initial bolus infusion of 0.04 mg/kg intravenously (i.v.), followed by additional doses of 0.5 mg i.v. midazolam, titrated to achieve a Ramsay sedation scale score of 3–4. Group D received dexmedetomidine with an initial bolus infusion of 1 mcg/kg/hr i.v. over 10 minutes, followed by a continuous infusion of 0.2–0.7 mcg/kg/hr, titrated to achieve an RSS of 3–4. A Mini Mental Status Examination (MMSE) was performed prior to sedation and in the recovery room once the Modified Aldrete Score (MAS) reached 9–10. Patient heart rates, arterial pressure and pain were evaluated.
Results:
Patients in Group D had lower heart rates at 20, 25, 30, 35 and 40 minutes following the initiation of sedation (p<0.05). There was no statistical difference in arterial pressure, RSS, MMSE or respiratory rate between the two groups. Coughing, nausea and vomiting occurred in 3 patients in Group M (12%), whereas no patient in Group D experienced these symptoms. The procedure elicited a gag response in 7 patients in Group M (28%) and in 4 patients in Group D (16%), with no significant difference between groups (p>0.05). When patient and surgeon satisfaction was compared between the two groups, Group D showed higher surgeon satisfaction scores (p<0.05).
Conclusion:
The use of dexmedetomidine for conscious sedation during short, invasive procedures, such as endoscopic retrograde cholangiopancreatography, could be a superior alternative to the use of midazolam.
Keywords: Conscious sedation, ERCP, Dexmedetomidine, Midazolam
Özet
Amaç:
Bu çalışmada endoskopik retrograd kolonjiopankreatografi işlemi sırasında bilinçli sedasyon için uygulanan midazolam ve deksmedetomidinin hemodinami ve solunum parametreleri ve yan etkiler açısından karşılaştırılmaları amaçlandı.
Gereç ve Yöntem:
18–80 yaşları arasında, Amerikan Anestezistler Birliği’nin (ASA) sınıflamasına göre I ve II anestezi risk grubuna giren 50 olgu çalışmaya alınarak, çalışma protokolü anlatıldı. Hastalar randomize olarak iki gruba ayrıldı: Grup M’deki olgulara midazolam 0.04 mg kg-1 intravenöz (i.v) uygulandıktan sonra RSS’u 3–4 olacak şekilde 0.5 mg ek dozlarla işleme devam edildi. Grup D’deki olgulara deksmedetomidin 1 μg kg-1 sa-1 olacak şekilde 10 dakika yükleme dozu uygulandı. Takiben olguların RSS’u 3–4 olacak şekilde 0.2–0.7 μg kg-1sa-1 deksmedetomidin infüzyonuna başlandı. Olguların demografik verileri ve çalışma süresince vital bulguları kaydedildi. Sedasyon skorları ölçümünde Ramsay sedasyon skoru kullanıldı. Mini mental test (MMT) olgulara sedasyon öncesinde ve derlenme odasında modifiye Aldrete skoru (MAS) 9–10 olduğunda uygulandı. Ağrı değerlendirilmesinde yüz ağrı ölçeği (YAÖ) kullanıldı.
Bulgular:
Grup D’de 20, 25, 30, 35, 40. dakikalarda ölçülen kalp hızı değerleri Grup P’ye göre daha düşük bulundu (p<0.05). Grupların kendi arasında ve grup içi karşılaştırılmasında, ortalama arter basıncı değerlerinde istatistiksel olarak anlamlı bir fark bulunmadı. Kognitif fonksiyonlar açısından iki grup karşılaştırıldığında istatistiksel olarak anlamlı farklılık saptanmadı. Komplikasyonlar açısından gruplar karşılaştırıldığında öksürük, bulantı ve kusma Grup M’de 3’er (%12) olguda görülürken, Grup D’de hiçbir olguda gözlenmedi. Öğürme Grup M’de 7 (%28), Grup D’de ise 4 (%16) olguda gözlendi, ancak istatistiksel farklılık saptanmadı (p>0.05). Gruplar arasında cerrah ve hasta memnuniyeti karşılaştırıldığında, Grup D’de cerrah memnuniyetinin daha yüksek olduğu saptandı (p<0.05).
Sonuç:
Endoskopik retrograd kolonjiopankreatografi ve benzeri kısa süreli invazif işlemlerde deksmedetomidin ile bilinçli sedasyon uygulaması rutinde kullanılan midazolam uygulamalarına önemli bir alternatif olabilir.
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) plays a crucial role in the diagnosis and treatment of pancreaticobiliary pathologies, and its use has increased in recent years. To provide patient comfort and facilitate the work of the surgeon, patients are given conscious sedation during the ERCP procedure [1]. The preferred anesthetic agent for conscious sedation is midazolam. Midazolam is a popular drug that is frequently used in day surgery because of its early onset activity, short duration of action, protective effects on cardiovascular stability, and the fact that the patient regains normal mental functions only four hours after intravenous application [2]. In recent years, dexmedetomidine has been used as an alternative to midazolam in conscious sedation applications. Because it provides sedation and analgesia but does not cause respiratory depression, dexmedetomidine is considered a suitable drug for operations that are performed under local anesthesia [3, 4].
The primary aim of this study was to compare the effects of midazolam and dexmedetomidine during ERCP on hemodynamic, respiratory, sedative and cognitive functions. Secondary outcomes were the degree of comfort experienced by patients and the usefulness of the drug to surgeons.
Materials and Methods
Following the approval of the Ethical Board and after obtaining informed consent from the patients, 50 sedation cases for diagnostic ERCP were included in this prospective, randomized, double-blind study.
The patients that received sedation for ERCP were evaluated preoperatively. A total of 50 patients, ranging in age from 18 to 80 years and classified in the 1–2 anesthesia risk group according to the American Society of Anesthesiologists (ASA), were enrolled in the study. The study protocol was explained to all of the patients. The following were excluded from the study: patients with difficulty communicating (due to language problems or deafness), patients who were allergic to the drugs used, patients with comorbid uncontrolled internal problems (such as diabetes mellitus, hypertension, and hepatic or renal insufficiency), patients with a central nervous system or psychiatric disease, patients with a history of long-term opioid use or alcohol abuse, and patients who were pregnant or suspected of pregnancy. The patients who did not receive pre-medication were given infusions of isotonic sodium chloride at a rate of 5 ml per minute via a 20-G intravenous catheter that was inserted into the left or right antecubital region. The patient demographic data were noted. Patient heart rates (HR), non-invasive systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), peripheral oxygen saturation (SpO2) and respiration rate (RR) were monitored. During the operations, all patients were given O2 by mask at a rate of 6 L/min. The Ramsay Sedation Scale (RSS; 1–6) was used to evaluate the depth of sedation, the Mini Mental State Examination (MMSE) was used to evaluate cognitive function, and the Modified Aldrete Score (MAS; 0–10) was used to assess recovery [5–7]. When the MAS were found to be between 9 and 10, MMSE was applied before sedation and in the recovery room. The Facial Pain Rating Scale (FPS; 0–10) was used to evaluate pain (Figure 1) [8]. An FPS evaluation was performed by the anesthesiologist at five-minute intervals throughout the procedure and in the recovery unit until aMAS value between 9 and 10 was reached.
Figure 1.
Faces pain rating scale (FPS).
For group M patients, following the application of a single dose of 0.04 mg/kg i.v. midazolam, additional 0.5-mg doses were administered until the RSS reached 3–4. For group D patients, dexmedetomidine was administered at a loading dose of 1 µg/kg/h, which was followed by a 0.2–0.7-µg/kg/h infusion until the RSS reached 3–4. All patients were given 1 µg/kg fentanyl at the beginning of the procedure. The vital parameters of the patients were recorded before and after the loading dose and every five minutes throughout the procedure. An SpO2 level below 92% for more than 10 seconds was considered oxygen desaturation. A heart rate under 50 beats per minute or a 20% decrease from the baseline was considered an indication of bradycardia, whereas a heart rate of over 110 or an increase in the baseline level of more than 20% was considered a tachycardia. Mean arterial pressure levels that were lower than 60 mmHg or 20% less than the baseline were regarded as hypotension, and a mean arterial pressure value of over 150 mmHg or a 20% increase from the baseline was regarded as hypertension. Possible complications, such as respiratory depression, allergies, coughing, gagging, nausea and vomiting, were recorded. FPS and RSS were recorded every five minutes. The satisfaction of both the surgeon and the patient were assessed. In the recovery room, MAS, FPS and RSS of the patients were recorded every five minutes by an anesthesiologist who had not been informed about the medications used. The cost of the drugs was calculated by determining the amount of drug used in milliliters.
The program SPSS for Windows 13.0 (Chicago, IL) was used for statistical analysis in the study. The continuous variables in the study were given as the mean, standard deviation, and maximum and minimum values. The Shapiro-Wilk test was used to determine the normality of the continuous variables. Comparisons of normally distributed continuous variables were performed using the parametric independent sample t-test. The Wilcoxon test was used for intragroup comparisons of dependent variables. For intergroup comparisons of dependent variables, the percentage changes of these variables from initial values were first calculated, and the values for the two groups were compared using the Mann-Whitney U test. The Pearson Chi-square and the Fisher’s exact chi-square tests were used to compare categorical variables. The results were considered significant when the p value was less than 0.05.
Results
There were no statistically significant differences in either the demographic data or the duration of sedation between the two groups (Table 1). There was also no significant difference in heart rate after the induction period in the intragroup comparison. The heart rates of the two groups were significantly different at 20, 25, 30, 35, and 40 minutes (p<0.05) (Figure 2).
Table 1.
Demographic data and sedation time
| Group M (n=25) | Group D (n=25) | |
|---|---|---|
| Gender F/M | 9/16 | 10/15 |
| Age (year) | 53.7±18.3 | 57.0±14.6 |
| Weight (kg) | 67.3±14.9 | 70.3±10.1 |
| Height (cm) | 163.3±8.6 | 162.0±8.9 |
| Sedation time (min) | 25.1±8.0 | 25.8±8.9 |
Data were given as n or mean±SD
Figure 2.
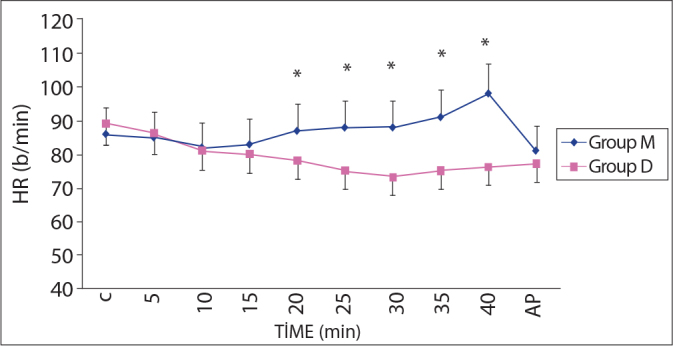
Heart Rates during procedure (mean ± SD).
*p<0.05; Group M compared to Group D
C: Control, AP: After procedure, HR: Heart rate.
Throughout the course of the operations, none of the patients experienced a bradycardia or tachycardia that warranted treatment. Intergroup and intragroup comparisons of the measurements that were taken after the induction period and during the operations relative to the control levels showed no significant differences (Figure 3). Blood pressure changes requiring treatment were not observed during the operations.
Figure 3.
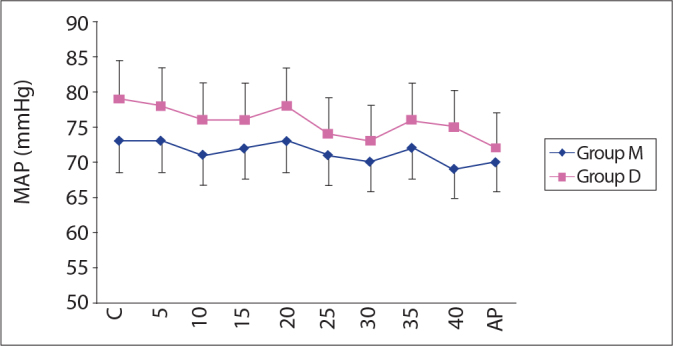
Mean arterial pressure (mean ± SD).
C: Control, AP: After procedure, MAP: Mean arterial pressure.
When the levels of surgeon and patient satisfaction were compared, Group D had higher surgeon and patient satisfaction scores (p<0.05 for both) (Table 2).
Table 2.
Comparing surgeon and patient satisfaction in groups
| Group M (n=25) | Group D (n=25) | |||
|---|---|---|---|---|
|
| ||||
| Satisfied | Very Satisfied | Satisfied | Very Satisfied | |
| Surgeon satisfaction (n) | 11 | 14 | 5 | 20* |
| Patient satisfaction (n) | 7 | 18 | 5 | 20* |
p<0.05; Group D vs Group P
When the groups were compared regarding the occurrence of complications, coughing, nausea and vomiting were observed in three patients in Group M (12%), but no patients in Group D experienced these complication. Gagging was experienced by seven patients in Group M (28%) and four patients in Group D (16%). Allergic responses did not occur in any patients. Serious bradycardia and hypotension were also not observed. The complications are listed in Table 3.
Table 3.
Complications according to groups
| Group M (n=25) | Group D (n=25) | |
|---|---|---|
| Coughing (n, %) | 3 (12) | 0 |
| Gagging (n, %) | 7 (28) | 4 (16) |
| Nausea and vomiting (n, %) | 3 (12) | 0 |
In the recovery room, 6 cases in Group M (24%) and 20 cases in Group D (80%) reached an MAS value of 10 in the fifth minute. By the tenth minute, 16 Group M patients (64%) and 24 Group D patients (96%) attained an MAS value of 10. There were statistically significant differences between the two groups at minutes five and ten (p<0.001 and p<0.05, respectively) (Figure 4).
Figure 4.
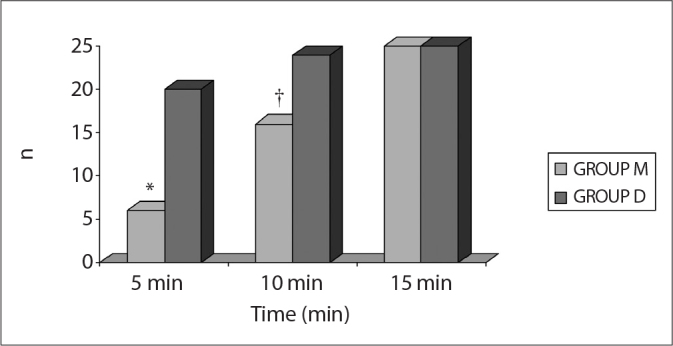
Number of patients who Aldrete score was 10 in PACU.
*p < 0.001; Group M compared to Group D
†p<0.05; Group M compared to Group D
There was no significant difference in preoperative MMSE values either within or between groups (Table 4).
Table 4.
Evaluation of cognitive functions (mean±SD)
| MMSE Baseline | MMSE in PACU | |
|---|---|---|
| Group M | 25.4 ±3.5 | 24.1±3.7 |
| Group D | 24.4±4.1 | 24.3±4.1 |
MMSE= Mini mental state examination
There were no significant differences in SpO2, RR, RSS and FPS values between groups during the procedures, after the procedures or during the follow-up period.
Discussion
ERCP plays a very important role in the diagnosis and therapy of pancreaticobiliary pathologies. Because it is performed orally via an endoscope, it is an extremely painful and irritating procedure when conducted without sedation [10].
To ensure immobility, sufficient analgesia, and the avoidance of coughing, gagging or nausea, patients should be sedated while undergoing ERCP procedures. It is advisable that the protective reflexes are not suppressed and that the surgeon can work comfortably. Therefore, a sufficient dose of a conscious sedation medication should be administered, and monitoring methods should be performed [11]. In our study, we ensured that the patients in each group were motionless but sufficiently conscious to cooperate. Although they were sedated, their protective reflexes remained intact.
Under conscious sedation, patients retain their protective reflexes, and they are generally able to go home after a few hours. Rapid recovery is an advantage not only for the patient but also for hospitals and day surgery units. Conscious sedation is the preferred practice for certain surgical interventions; one advantage that it has over general anesthesia is that patient-doctor cooperation is possible. The reductions in anxiety and amnesia also result in higher levels of patient comfort [12].
During ERCP operations, the appropriate drug and level of sedation that is required for conscious sedation are different for each patient. The choice of drug and sedation level should be made according to the patient’s age and general health status and the experience of the surgeon and anesthesiologist. A have few adverse effects, should depress the awareness level of the patient in a controlled manner, should prevent the suppression of protective reflexes, should not depress respiration, and should enable rapid and complete recovery after the procedure. Furthermore, its metabolites should be inactive, and it should not lead to resedation [13, 14].
The doses of midazolam and dexmedetomidine that we used were able to preserve sufficient consciousness to allow communication but provided the necessary degree of sedation to enable surgical comfort and an adequate quality of recovery (specifically in terms of their inactive metabolites and the absence of resedation).
In previous studies that used conscious sedation, different doses of midazolam were used. Habib et al. [2] administered a single dose of midazolam (0.015 mg/kg i.v.), and Mc Hardy et al. [15] administered a single dose of midazolam (0.015 mg/kg i.v.). In the present study, we administered a single dose of 0.04 mg/kg midazolam i.v. prior to the ERCP procedures. This drug administration was followed by additional 0.5-mg doses to keep the RSS at 3–4.
In studies that used sedation for interventions that were performed under local and regional anesthesia, different doses of dexmedetomidine were used. Arain et al. [16] administered dexmedetomidine in a 1-µg/kg initial dose for 10 minutes and then continued with a 0.4-µg/kg/h maintenance dose for intraoperative sedation. During regional anesthesia in patients undergoing carotid endarterectomy, McCutheon et al. [17] began with dexmedetomidine at 0.5 µg/kg for 5 minutes and continued with 0.2 µg/kg/h. By contrast, Ibacache et al. [18] used a single dose of 0.3 µ/kg i.v. dexmedetomidine for 10 minutes to sedate and reduce agitation in children undergoing sevoflurane anesthesia. In our study, we administered a 1-µg/kg loading dose of dexmedetomidine for 10 minutes prior to the ERCP procedures. To maintain the RSS at 3–4, this dose was followed by an infusion at 0.5–0.7 µg/kg/h. Using these doses, we were able to maintain adequate sedation with no negative effects on hemodynamics, respiratory parameters or recovery scores.
In conclusion, administration of a 1-µg/kg loading dose of dexmedetomidine followed by a 0.5–0.7-µg/kg/h infusion can provide effective sedation with no negative effects on hemodynamic or respiratory parameters. Therefore, dexmedetomidine could be an important alternative to midazolam for conscious sedation in ERCP and other short-duration invasive procedures, because it has a shorter recovery time and minimal complications. Therefore, the effectiveness of dexmedetomidine for conscious sedation in minimally invasive procedures other than ERCP should be investigated.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Chen WX, Lin HJ, Zhang WF, Gu Q, Zhong XQ, Yu CH, et al. Sedation and safety of propofol for therapeutic endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2005;4:437–40. [PubMed] [Google Scholar]
- 2.Habib NE, Mandour NM, Balmer HG. Effects of midazolam on anxiety level and pain perception in cataract surgery with topical anesthesia. J Cataract Refract Surg. 2004;30:437–43. doi: 10.1016/S0886-3350(03)00557-1. [DOI] [PubMed] [Google Scholar]
- 3.Aantaa R, Jaakola ML, Kallio A. A comparison of deksmedetomidine, an alpha2-adrenoceptor agonist, and midazolam as i.m. premedication for minor gynaecological surgery. Br J Anaesth. 1991;67:402–9. doi: 10.1093/bja/67.4.402. [DOI] [PubMed] [Google Scholar]
- 4.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxolone. Br Med J. 1974;12:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein S, Mc Hugh PR. ‘Mini Mental State’ A practical method for grading the cognitive state of patients for the patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Alderette JA, Kroulik DA. Postanesthetic recovery score. Anesth Analg. 1970;51:543–6. [PubMed] [Google Scholar]
- 8.Talu GK. Ağrılı hastanın değerlendirilmesi. In: Erdine S, editor. Ağrı. 3rd edition. İstanbul: Nobel kitabevleri; 2007. pp. 61–9. [Google Scholar]
- 9.American Society of Anesthesiologists Task Force on Sedation Analgesia by Non Anesthesiologists. Practice guidelines for sedation and analgesia by Non anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Riphaus A, Stergiou N, Wehrmann T. Sedation with propofol for routine ERCP in high-risk octogenarians: A randomized, controlled study. Am J Gastroenterol. 2005;100:1957–63. doi: 10.1111/j.1572-0241.2005.41672.x. [DOI] [PubMed] [Google Scholar]
- 11.Demiraran Y, Korkut E, Tamer A, Yorulmaz I, Kocaman B, Sezen G, et al. The comparasion of dexmedetomidine and midazolam used for sedation of patients during upper endoscopy: A prospective, randomized study. Can J Gastroenterol. 2007;21:25–9. doi: 10.1155/2007/350279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweickert WD, Kress JP. Strategies to optimize analgesia and sedation. Crit Care. 2008;12:6–16. doi: 10.1186/cc6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie N. Sedation during regional anaesthesia: Indications, advantages and methods. Eur J Anaesthesiol. 1996;13:2–7. doi: 10.1097/00003643-199607001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kenny GN. Patient sedation: technical problems and developments. Eur J Anaesthesiol. 1996;13:18–21. doi: 10.1097/00003643-199607001-00005. [DOI] [PubMed] [Google Scholar]
- 15.McHardy FE, Fortier J, Chung F, Krishnathas A, Marshall SI. A comparasion of midazolam, alfentanil and propofol for sedation in oupatient intraoculer surgery. Can J Anesth. 2000;47:214–21. doi: 10.1007/BF03018914. [DOI] [PubMed] [Google Scholar]
- 16.Arain SR, Ebert TJ. The effcacy, side effects and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 17.McCutcheon CA, Orme RM, Scott DA, Davies MJ, McGlade DP. A comparison of dexmedetomidine versus convantional therapy sor sedation and hemodynamic control during carotid endarterectomy performed under regional anesthesia. Anesth Analg. 2006;102:668–75. doi: 10.1213/01.ane.0000197777.62397.d5. [DOI] [PubMed] [Google Scholar]
- 18.Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane in children. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]


