Abstract
Eosinophilic pneumonia (EP) is a rare disease of the lung. We aimed to present atypical course of two EP cases. They were admitted to our hospital because of acute respiratory distress syndrome (ARDS) in postpartum period. Eosinophilia was detected in bronchoscopic bronchoalveolar lavage and laboratory examination. In these cases, no spesific cause for eosinophilic pneumonia was determined and steroid treatment was started. After the treatment, the patients were in full recovery which were confirmed by clinical and radiological investigations, readmitted to our clinic with relapses of ARDS. The patients have received regular treatment for 1 year. Our cases were neither fitting the classic definitions of acute eosinophilic pneumonia nor chronic eosinophilic pneumonia. Therefore, we wanted to contribute additional data in the literature by sharing these interesting cases.
Keywords: Eosinophilia, Postpartum period, Respiratory distress syndrome
Özet
Eozinofilik pnömoniler akciğerin nadir hastalıklarıdır. Biz, atipik seyir gösteren iki eozinofilik pnömoni vakasını sunmayı amaçladık. Vakalar, hastanemize postpartum periodda akut solunum yetmezliği (ASY) nedeniyle kabul edildiler. Laboratuar muayenesinde ve bronkoskopik bronkoalveolar lavajda eozinofili tespit edildi. Eozinofili nedeni belirlenemeyen hastalara steroid tedavisi başlandı. Tedavi sonrası tam klinik ve radyolojik yanıt alınan hastalar, kliniğimize ASY nüksleriyle tekrar kabul edildiler. Hastalar, 1 yıl düzenli tedavi aldılar. Vakalarımızın, klinik tanımlamaları ne akut eozinofilik pnömoni ne de kronik eozinofilik pnömoniye uymaktadır. Bu nedenle, bu iki ilginç olguyu literatürle paylaşmak istedik.
Introduction
The term of eosinophilic lung disease describes a group of entities characterized by accumulation of eosinophils in the pulmonary interstitium and airspaces. They are classified as eosinophilic lung disease of unknown cause, eosinophilic lung disease of known cause which is mainly originating from infectious and drug and lung diseases with possible associated eosinophilia [1–3]. Chronic eosinophilic pneumonia (CEP) and acute eosinophilic pneumonia (AEP) are two of the unknown causes. We aimed to present atipical course of two eosinophilic pneumonia cases who were admitted to our hospital because of ARDS and showed relapses in postpartum period.
Case Reports
Case 1: A 32-year-old female admitted to the hospital with complaints of progressive dyspnea, non-productive cough, malaise and myalgias. The onset of her symptoms was immediately after spontaneous vaginal delivery without complications twenty-five days ago. Her medical history was unremarkable except for an occasional cough for two years. She was non-smoker and had no history of alcohol consumption or use of any medications. Her physical examination revealed a pulse rate of 146 beats/min, blood pressure 90/60 mmHg, respiratory rate of 40 breaths/min, and an arterial oxygen saturation of 70% while breathing ambient air. Her arterial blood gas sample PaO2 was 40 mmHg (FiO2; 0,21). On admission, laboratory examination of the patient showed peripheral eosinophilia (1100/mm3). Chest X-ray and computed tomography (CT) scan of the thorax showed bilateral homogenous consolidation with subpleural predominance in upper and middle zones (Figure 1a,1b). Shortly after her presentation, the patient underwent endotracheal intubation for hypoxic respiratory failure (PaO2 70 mmHg, FiO2 of 100%). The patient was diagnosed as acute respiratory distress syndrome and required mechanic ventilation (IPPV-PEEP) for 5 days. Then, the patient was extubated and admitted to the medical respiratory unit. The patient was initiated steroid (prednisone) treatment (1 mg/kg per day i.v.) and continued later. The patient was discharged after stabilization on 30 mg of oral prednisolone treatment. On day 9 after discharging, the patient re-admitted to the hospital because of the relapse of ARDS. The patient had discontinued steroid treatment after discharge. The patient was required mechanical ventilation again due to ARDS. In the intensive care unit, the patient underwent bronchoscopy with the suspicion of AEP before re-initiating steroid treatment. Special stains and cultures for mycobacteria, bacteria, fungi, and opportunistic organism were negative and the bronchoalveolar lavage (BAL) revealed 31% eosinophils. Stool cultures were negative for ova and parasites and antineutrophilic cytoplasmic antibodies were negative. The steroid treatment started again and clinical and radiologic response was observed in one day period.
Figure 1.
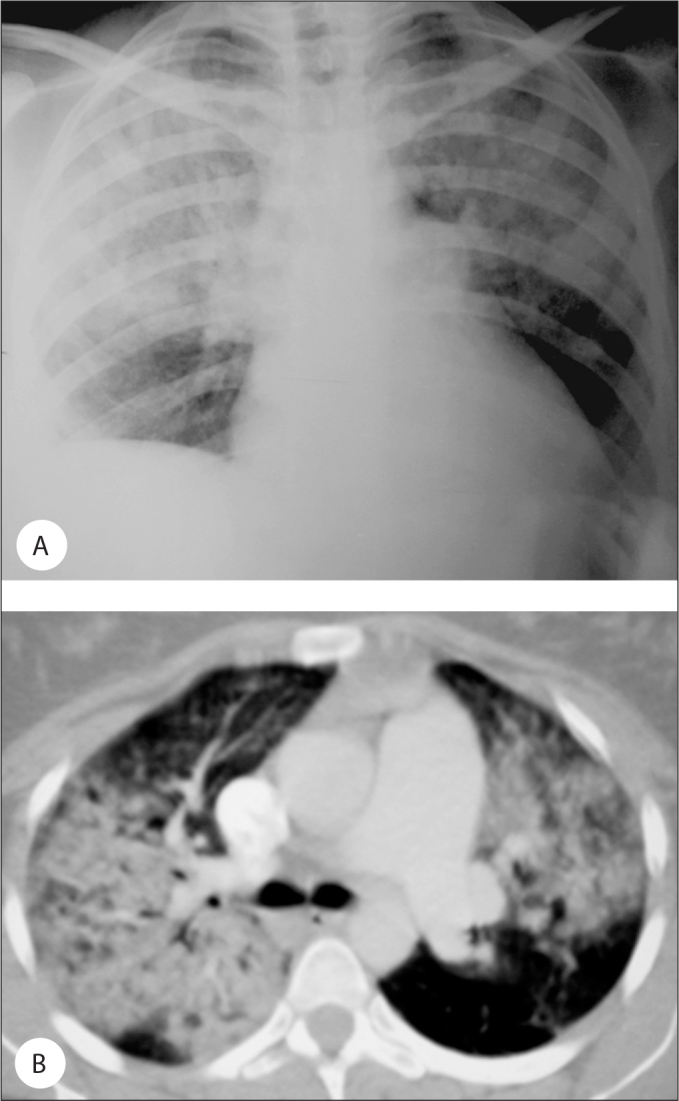
A) The patient’s chest X-ray on admission, B) The patient’s computed tomography scan of the thorax on admission.
Case 2: A 33 year-old-female admitted to the hospital with the complaints of progressive dyspnea, non-productive cough and hemoptysis. The onset of her symptoms was immediately after spontaneous vaginal delivery without complications twenty days ago as Case 1. Her medical history was unremarkable except for a history of cough for three years. She was non-smoker and had no history of alcohol consumption. She was on inhalation therapy because of cough for one year but she did not use her drugs regularly.
On physical examination, a pulse rate of 155 beats/min, blood pressure of 90/50 mmHg, respiratory rate of 45 breaths/min, and an arterial oxygen saturation of 65% while breathing ambient air was noted. Her arterial blood sample PaO2 was 34mmHg (FiO2; 0.21). Chest X-ray and CT scan of the thorax revealed the same findings as in Case 1 (Figure 1a, b). The patient was diagnosed as ARDS. Shortly after presentation, the patient underwent endotracheal intubation for hypoxic respiratory failure. An arterial blood gas sample was obtained after intubation and on a FiO2 of 100% revealed a partial pressure of oxygen of 85 mmHg, confirming the diagnosis of the ARDS. Because of the first case, the patient’s admission laboratory data were investigated carefully and we saw that the patient had peripheral eosinophilia (1600/mm3). BAL was obtained by bronchoscopy in intensive care unit. The BAL revealed 42% eosinophils and all other laboratory datas were negative as in Case 1. The patient was treated with methylprednisolone. In two days, clinical and radiologic improvement was observed. The patient was discharged with 30 mg of oral prednisolone. After discharge, the patient re-admitted to the hospital because of ALI/ARDS for three times. The first relapse, as ARDS, occured fifteen days later. The others were observed as ALI in the following two months. In this case, no spesific cause for eosinophilic pneumonia was determined.
Discussion
Both of our cases were postpartum eosinophilic pneumonias of undetermined origin. Postpartum eosinophilic pneumonia has been previously reported [2–5]. Those cases in the literature are idiopathic chronic eosinophilic pneumona cases presenting after post-partum period with or without relapses. However, our cases are very unique ones because of their acute presentation of acute repiratory distress and showing relapses, even as repiratory failure, during the course of the disease. Thus, our cases are neither fitting the classic definitions of acute eosinophilic pneumonia nor chronic eosinophilic pneumonia.
Idiopathic acute and chronic eosinophilic pneumonias diagnostic criteria have been described [1]. In the literature review, there were some cases, all were CEP, associated with preganancy or postpartum period [2–5]. Tosini et al. have repoted a 24-year-old pregnant case with CEP whose symptoms during pregnancy have been controlled by increasing steroid dose [2]. In the report by Tohya et al., a 28-year-old pregnant case who had secondary peripheral eosinophilia and pulmonary infiltration following antibiotic use has been described. Although symptoms of the cases have been resolved spontaneously, her symptoms have relapsed on the sixth month of postpartum follow-up period and required steroid use [3]. In another case by Dothager and Kollef, a 22-year-old case who had the typical symptoms of CEP on sixth month of pregnancy has been reported. In this case, symptoms have continued in the postpartum period and diagnosis has been confirmed via BAL analysis. Her symptoms has improved with steroid treatment and no relapse has occured [4]. Only one case with idiophatic eosinophilic pneumonia, a 23-year-old female, by Davies et al., in the literature is associated with previous asthma and has developed two relapses on sixth weeks of her pregnancy. She has been treated with steroid treatment and no relapses occurred [5].
In comparison to the previous cases, our cases were a decade older. They had no previous diagnosis and medication use during or before pregnancy, had acute onset of illness with more severe symptoms as acute respiratory distress syndrome and more relapses in the course of postpartum period. Although severity of the disease and relapses of the cases, they responded well to the steroid treatment. Presence of previous cough history in our cases suggested that they may have a cough variant asthma. The severity of their illness may be associated the under-diagnosed and untreated asthma. In our cases, asthma diagnosis was uncertain and they had more relapses. The fact that they did not take any asthma medication may be the reason of relapse. The association between asthma and chronic eosinophilia is well known and in cases with asthma and chronic eosinophilia, the diseases affect each other [6]. For example, asthma may be relatively severe and got worse after the diagnosis of CEP. On the contrary, presence of asthma at the time of diagnosis CEP may be associated with less relapses of CEP, possibly because of a higher frequency of long-term inhaled corticosteroids use in asthmatics.
No increase of symptoms in postpartum period has been reported except one case report [5]. The decreased cortisol levels, which increase in pregnancy, may have role in the increase of sympthoms in postpartum period. However, in our cases, the most reasonable explanation for relapses was their poor compliance to the steroid treatment.
As a conclusion, these are unique cases of chronic eosinophilic pneumonia in the literature. The reason of these cases presenting as severe clinical manifestation may result from an undiagnosed asthma. In the cases of acute repiratory distress syndrome, especially in the post-partum period and who have previous history of respiratory symptoms, eosinophilic pneumonias should be taken into consideration.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Cordier JF, Cottin V. Eosinophilic Lung Disease. In: Albert RK, Spiro SG, Jett JR, editors. Clinical Respiratory Medicine. 3rd Edn. Mosby Elsevier; Philadelphia: 2008. pp. 681–7. [Google Scholar]
- 2.Tosini C, Faden D, Cattaneo R, et al. Idiopathic eosinophilic pneumonia and pregnancy: report of a case. Int Arch Allergy Immunol. 1995;106:173–4. doi: 10.1159/000236841. [DOI] [PubMed] [Google Scholar]
- 3.Tohya T, Matsui K, Itoh M, Okamura H. A case of pulmonary infiltration with eosinophilia syndrome in pregnancy. Acta Obstet Gynaecol Jpn. 1990;42:389–92. [PubMed] [Google Scholar]
- 4.Dothager DW, Kollef MH. Postpartum pulmonary infiltrates with peripheral eosinophilia. Chest. 1991;99:463–4. doi: 10.1378/chest.99.2.463. [DOI] [PubMed] [Google Scholar]
- 5.Davies HCW, Mackinlay CI, Wathen CG. Recurrent post-partum pulmonary eosinophilia. Thorax. 1997;52:1095–6. doi: 10.1136/thx.52.12.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchand E, Etienne-Mastroianni B, Chanez P, et al. Idiopathic chronic eosinophilic pneumonia and asthma: how do they influence each other? Eur Respir J. 2003;22:8–13. doi: 10.1183/09031936.03.00085603. [DOI] [PubMed] [Google Scholar]


