Abstract
Imatinib mesylate (STI 571) is one of the fundamental chemotherapeutic agents used in the treatment of the chronic, accelerated and blastic phases of chronic myelocytic leukemia (CML), gastrointestinal stromal tumors and Philadelphia chromosome-positive acute lymphoblastic leukemia. It selectively inhibits receptor tyrosine kinases. Its effects limit the use of this drug. We present a case with a serious skin reaction requiring the discontinuation of the drug and that developed in relation to imatinib therapy. Six months prior, a 61-year-old male patient presenting to the hematology polyclinic with complaints of weight loss and sweating was hospitalized due to high leukocyte value. As a result of the hemogram, biochemistry analyses, peripheral blood smear examination, bone marrow aspiration evaluation, cytogenetic examination using FISH and PCR that were performed, CML was diagnosed. Additionally, to exclude myelofibrosis, we examined a bone marrow biopsy. Imatinib mesylate was started at 400 mg/day orally. In the fourth month of treatment, the patient complained of itching and a skin rash. Although the drug dose was reduced (300 mg/day), his complaints gradually increased. The skin biopsy result was superficial perivascular dermatitis. Imatinib was discontinued, and the patient was started on corticosteroid. The lesions disappeared completely. A month later, the patient was restarted on imatinib mesylate. However, the lesions recurred more prominently. His itching increased. The patient was considered intolerant to imatinib mesylate, and a second-generation tyrosine kinase inhibitor, dasatinib 100 mg/day, was started orally. The follow-up and treatment continues for the patient, who has been taking dasatinib 100 mg/day for the last two months without any skin finding or complaints. Imatinib mesylate–induced skin reactions are associated with the pharmacologic effect of the drug rather than hypersensitivity to the drug. Skin reactions are frequently observed, and this side effect is dose dependent. However, the interesting aspect of our case was that despite dose reduction, skin findings gradually increased, and eventually the drug had to be discontinued.
Keywords: Chronic myelocytic leukemia (CML), Imatinib, Skin lesions
Özet
İmatinib mesilat (STI 571); kronik miyelositer lösemi (KML)’nin kronik, akselere ve blastik fazı, gastrointestinal stromal tümörler ve philadelphia pozitif akut lenfoblastik lösemi tedavisinde kullanılan temel kemoterapötik ajanlardan biridir. Selektif olarak tirozin reseptör kinazı inhibe eder. Bu ilaca bağlı gelişen yan etkiler ilacın kullanımını sınırlar. Biz imatinib tedavisine bağlı gelişen ve ilacın kesilmesini gerektiren ciddi cilt reaksiyonu olan bir olguyu sunduk. Altı ay önce hematoloji polikliniğine kilo kaybı ve terleme şikayeti ile başvuran 61 yaşındaki erkek hasta lökosit değerinin yüksek olması nedeniyle yatırıldı. Yapılan hemogram, biokimya tetkikleri, periferik kan yaymasının incelenmesi, kemik iliği aspirasyon ve biopsisinin değerlendirilmesi, FİSH ve PCR ile sitogenetik inceleme sonucu KML tanısı konuldu. Aynı zamanda miyelofibrozisi dışlamak için kemik iliği biopsi incelemesi yapıldı. İmatinib mesilat 400 mg/gün oral olarak başlandı. Tedavinin 4. ayında hastada ciltte kaşıntı ve döküntü şikayeti oldu. İlaç dozu azaltılmasına rağmen (300 mg/gün) şikayetleri giderek arttı. Cilt biyopsi sonucu yüzeyel perivasküler dermatit olarak geldi. İmatinib kesildi ve hastaya kortikosteroid başlandı. Lezyonlar tamamen kayboldu. Bir ay sonra hastaya tekrar imatinib mesilat başlandı. Ancak lezyonlar daha belirgin bir şekilde tekrarladı. Kaşıntısı arttı. Bunun üzerine hasta imatinib mesilat adlı ilaca intoleran olarak kabul edildi ve ikinci jenerasyon tirozin kinaz inhibitörü olarak dasatinib 100 mg/gün oral olarak başlandı. Son iki aydır dasatinib 100 mg/gün almakta olan hastanın herhangi bir cilt bulgusu ve şikayeti olmaksızın takip ve tedavisi devam etmektedir. İmatinib mesilata bağlı cilt reaksiyonları ilaca hipersensitiviteden ziyade ilacın farmakolojik etkisi ile ilişkilidir. Cilt reaksiyonları sık olarak gözlenir ve bu durum doz bağımlıdır. Ancak bizim olgumuzun ilginç tarafı doz azaltılmasına rağmen cilt bulgularının giderek artması ve en sonunda da ilacın kesilmek zorunda kalınması idi.
Case Report
A 61-year-old male patient presented to the hematology polyclinic 6 months prior to this report with complaints of weight loss and sweating. Upon presentation, the hemogram values were leukocytes: 66.800/µL; hemoglobin: 12.2 g/dL; neutrophil rate: 88.8%; and neutrophil count: 59.300 /µL. A peripheral blood smear was performed and analyzed. In the smear, a left shift and myeloid series dominance was detected. Bone marrow aspiration and biopsy were performed. In the bone marrow biopsy, there was a granulocytic series dominance. In the bone marrow aspiration, myelogranulocytic series had increased in prevalence (82%). Blast was not detected. The bone marrow was sent for a cytogenetic analysis. The BCR/ABL value, determined via polymerase chain reaction (PCR), was positive (0.00448965517). In the cytogenetic examination performed using fluorescence in situ hybridization (FISH), a BCR/ABL fusion signal was detected in 10% of the cells. The patient was diagnosed with CML. Imatinib mesylate was started at 400 mg/day orally. In the fourth month of treatment, the patient complained of itching and a skin rash. Although the drug dose was reduced (300 mg/day), his complaints gradually increased. We observed parchment paper-like annular lesions with a diameter of up to 1 cm in the middle section of the frontal region following the hairline; xerotic areas extending from the periumbilical region to the lumbar region; pigmented macular lesions observed mainly at distal locations in both arms and forearms and the lower extremities; shiny erythematous macules that became pale when pressed, with dispersed localization without clear boundaries, though more erythematous in the lines of the palm on the bilateral palmar surfaces of the hands; and purplish, dispersed macular rashes that became pale when pressed on the bilateral dorsal surfaces of the hands (Figure 1 and 2). The skin lesions were considered grade 3 according to the criteria of National Cancer Institute (NCI) (Table 1) [1].
Figure 1.
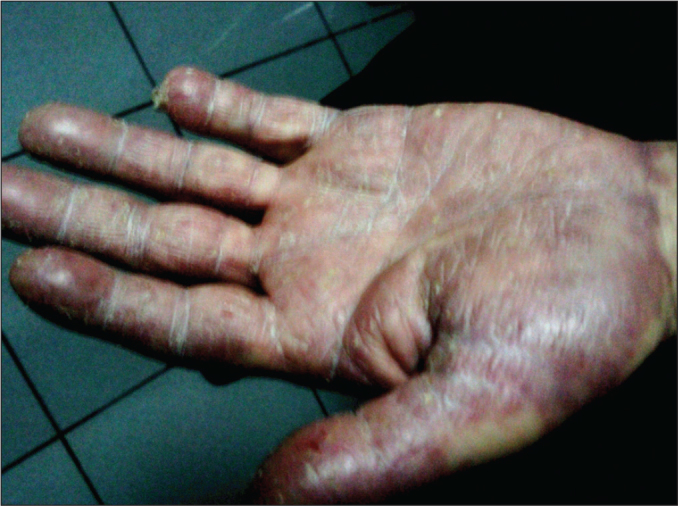
Erythematous-macular lesions on hand palmar surface.
Figure 2.
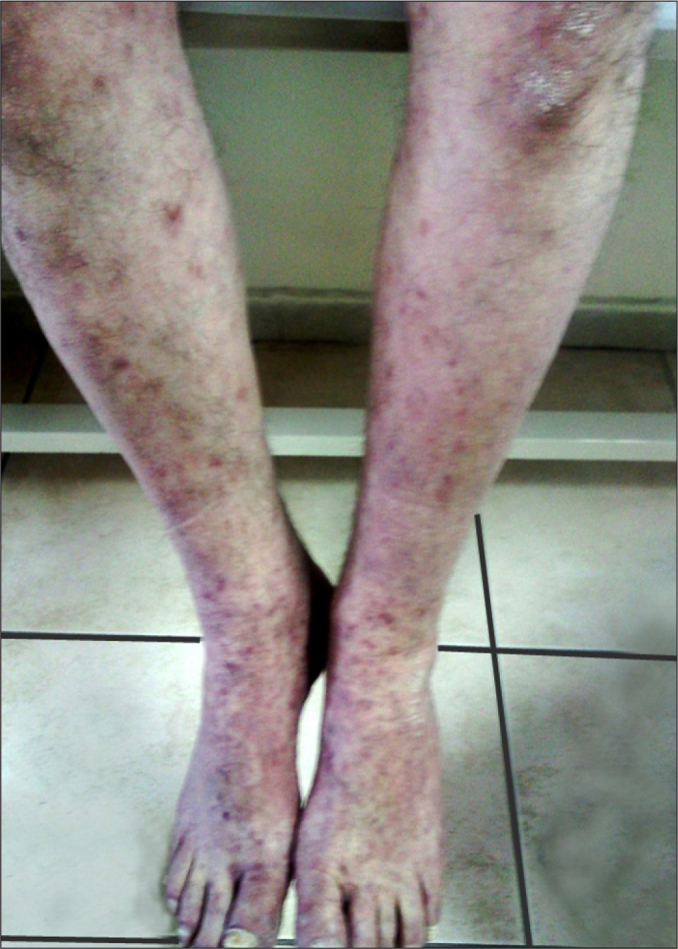
Macular lesions on frontal dorsal of both lower extremities.
Table 1.
Adverse skin reactions according to the National Cancer Institute (NCI) criteria [7]
| Skin Lesions | |
|---|---|
| Grade 1 | Macular or papular eruption or erythema without associated symptoms |
| Grade 2 | Pruritic macular or papular eruption or erythema with covering less than 50% of body surface area |
| Grade 3 | Symptomatic, generalized erythroderma or macular, papular or vesicular eruption, or desquamation covering more than 50% of body surface area |
| Grade 4 | Generalized exfoliative dermatitis or ulcerative dermatitis |
With the recommendation of dermatologists, a skin biopsy was obtained from the patient and indicated that this condition was associated with the imatinib mesylate. The skin biopsy result showed superficial perivascular dermatitis (Figure 3). Imatinib was discontinued, and the patient was started on corticosteroid 1 mg/kg/day. The lesions disappeared completely. A month later, the patient was restarted on imatinib mesylate. However, the lesions recurred more prominently. His itching increased. At the end of the 3rd month of this round of imatinib therapy, the patient achieved a hematological response but no cytogenetic or molecular response. Despite the response, the patient was considered intolerant to imatinib mesylate. Therefore, a second-generation tyrosine kinase inhibitor, dasatinib 100 mg/day, was started orally. The patient’s follow-up and treatment continues without any skin findings or complaints.
Figure 3.
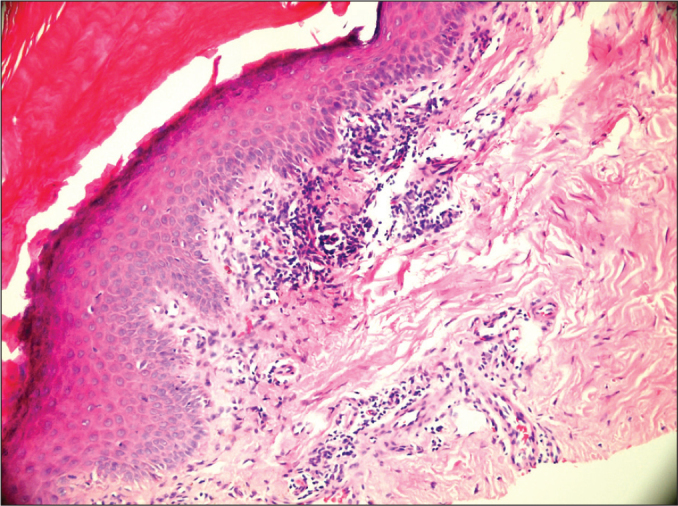
Microscopic image of the skin biopsy.
Discussion
Imatinib mesylate (STI 571) is a selective BCR-ABL tyrosine kinase inhibitor. It is the gold-standard therapy for chronic myelocytic leukemia [2]. Skin rashes are a possible side effect of imatinib therapy, and they are observed more frequently in women. In many cases, the lesions are mild, limited and may easily be treated with an antihistaminic or topical steroid. However, in serious cases, the use of a short-term oral steroid may be required [3]. As there was a severe skin reaction in our case, in addition to discontinuing imatinib, oral steroid therapy was commenced.
Imatinib-associated rashes are often pruritic. Lesions commonly arise in the form of erythematous or maculopapular lesions. They are generally observed in the forearm or body, and less frequently on the face. Skin biopsy may typically establish a toxic drug reaction [3].
Skin rashes may frequently be associated with the intake of imatinib (66.7%) [4]. However, grade 3 or 4 skin rashes are observed in only a small proportion of these cases (3.8%). In some patients, serious reactions may be observed in which the flaking of the skin occurs, and Stevens-Johnson syndrome has been reported [5, 6]. In such cases, imatinib should be discontinued urgently, and systemic 1 mg/kg/day steroid therapy should be commenced. In serious skin lesions that are resistant to supportive therapies, imatinib therapy should be discontinued. However, this situation is observed in fewer than 1% of all patients who take imatinib [3].
The standard treatment of skin rashes related to drug intake generally involves discontinuation of the suspected drug. On the other hand, as imatinib is an essential drug in CML therapy, many hematologists and oncologists continue with imatinib by reducing the dose or resume the drug once the skin reactions resolve. There are various approaches to serious skin reactions, such as temporary discontinuation of imatinib, administration of imatinib 1 day a week, administration of low-dose imatinib with a short-term oral steroid or administration of low-dose imatinib without steroids [6–8].
Imatinib-related skin reactions may be associated with the dosage. This is more often associated with the pharmacologic effect of imatinib [1]. However, the cause of skin rashes is not fully known. On the other hand, platelet-derived growth factor receptor (PDGFR), which is abundant in keratinocytes, is inhibited by imatinib, and this inhibition may play a significant role in the occurrence of skin reactions [9]. Some other studies suggest that some imatinib-related skin reactions are due to the inhibition of KIT. However, it is not completely clear why serious skin reactions related to imatinib intake only occur in a small proportion of these patients [3, 10].
Skin reactions related to imatinib intake are dose dependent. As the imatinib dose increases, the skin reactions and their severity also increase (Table 2). In one study, serious or life-threatening skin lesions were observed in those taking 600 mg/day or more of imatinib. These were not observed in anyone receiving 500 mg/day or less. This finding suggests that skin reactions are associated with the pharmacological effect of imatinib rather than hypersensitivity [11–13].
Table 2.
| Severity of the Rash | 25–140 mg/day Imatinib (n=14) | 200–300 mg/day Imatinib (n=31) | 350–500 mg/day Imatinib (n=37) | 600–1000 mg/day Imatinib (n=61) |
|---|---|---|---|---|
| Mild or moderate | 7% | 13% | 16% | 21% |
| Serious or life threatening | 0% | 0% | 0% | 5% |
Our case is presented here because grade 3 skin reactions related to imatinib intake are rarely observed, an imatinib-related grade 3 skin reaction has been previously reported in a male patient, our patient’s reaction was not dose dependent, his skin reaction occurred at a dose of 400 mg/day, and the skin reaction was exacerbated despite dose reduction. Furthermore, it was interesting that there was no skin reaction related to the second-generation tyrosine kinase inhibitor dasatinib, which was commenced after intolerance to imatinib was diagnosed.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201–6. doi: 10.1067/mjd.2003.44. [DOI] [PubMed] [Google Scholar]
- 2.Mahapatra M, Mishra P, Kumar R. Imatinib-induced Stevens-Johnson syndrome: recurrence after re-challenge with a lower dose. Ann Hematol. 2007;86:537–8. doi: 10.1007/s00277-007-0265-y. [DOI] [PubMed] [Google Scholar]
- 3.Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637–47. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 4.Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137:765–70. [PubMed] [Google Scholar]
- 5.Ferraresi V, Catricalà C, Ciccarese M, Ferrari A, Zeuli M, Cognetti F. Severe skin reaction in a patient with gastrointestinal stromal tumor treated with imatinib mesylate. Anticancer Res. 2006;26:4771–4. [PubMed] [Google Scholar]
- 6.Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117:620–2. doi: 10.1046/j.1365-2141.2002.03499.x. [DOI] [PubMed] [Google Scholar]
- 7.Rule SA, O’Brien SG, Crossman LC. Managing cutaneous reactions to imatinib therapy. Rule SA, O’Brien SG, Crossman LC. Blood. 2002;100:3434–5. doi: 10.1182/blood-2002-08-2431. [DOI] [PubMed] [Google Scholar]
- 8.Milojkovic D, Short K, Salisbury JR, Creamer D, du Vivier AW, Mufti GJ. Dose-limiting dermatological toxicity secondary to imatinib mesylate (STI571) in chronic myeloid leukaemia. Leukemia. 2003;17:1414–6. doi: 10.1038/sj.leu.2402991. [DOI] [PubMed] [Google Scholar]
- 9.Tanvetyanon T, Nand S. Overcoming recurrent cutaneous reactions from imatinib using once-weekly dosing. Ann Pharmacother. 2003;37:1818–20. doi: 10.1345/aph.1D184. [DOI] [PubMed] [Google Scholar]
- 10.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–25. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 12.Brouard MC, Prins C, Mach-Pascual S, Saurat JH. Acute generalized exanthematous pustulosis associated with STI571 in a patient with chronic myeloid leukemia. Dermatology. 2001;203:57–9. doi: 10.1159/000051705. [DOI] [PubMed] [Google Scholar]
- 13.Brouard M, Saurat JH. Cutaneous reactions to STI571. N Engl J Med. 2001;345:618–9. doi: 10.1056/NEJM200108233450814. [DOI] [PubMed] [Google Scholar]


