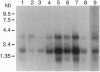
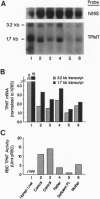
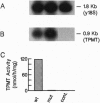
Abstract
Thiopurine S-methyltransferase (TPMT; S-adenosyl-L-methionine:thiopurine S-methyltransferase, EC 2.1.1.67) activity exhibits genetic polymorphism, with approximately 0.33% of Caucasians and African-Americans inheriting TPMT deficiency as an autosomal recessive trait. To determine the molecular genetic basis for this polymorphism, we cloned the TPMT cDNA from a TPMT-deficient patient who had developed severe hematopoietic toxicity during mercaptopurine therapy. Northern blot analysis of RNA isolated from leukocytes of the deficient patient demonstrated the presence of TPMT mRNAs of comparable size to that in subjects with high TPMT activity. Sequencing of the mutant TPMT cDNA revealed a single point mutation (G238-->C), leading to an amino acid substitution at codon 80 (Ala80-->Pro). When assessed in a yeast heterologous expression system, this mutation led to a 100-fold reduction in TPMT catalytic activity relative to the wild-type cDNA, despite a comparable level of mRNA expression. A mutation-specific PCR amplification method was developed and used to detect the G238-->C mutation in genomic DNA of the propositus and her mother. This inactivating mutation in the human TPMT gene provides insights into the genetic basis for this inherited polymorphism in drug metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Elion G. B. The purine path to chemotherapy. Science. 1989 Apr 7;244(4900):41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Horner M., Chu Y. Q., Kalwinsky D., Roberts W. M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991 Dec;119(6):985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- Grant D. M. Molecular genetics of the N-acetyltransferases. Pharmacogenetics. 1993 Feb;3(1):45–50. doi: 10.1097/00008571-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Heim M., Meyer U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990 Sep 1;336(8714):529–532. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- Honchel R., Aksoy I. A., Szumlanski C., Wood T. C., Otterness D. M., Wieben E. D., Weinshilboum R. M. Human thiopurine methyltransferase: molecular cloning and expression of T84 colon carcinoma cell cDNA. Mol Pharmacol. 1993 Jun;43(6):878–887. [PubMed] [Google Scholar]
- Jin A., Zhang Y., Xia Y., Traylor E., Nelson M., Van Etten J. L. New restriction endonuclease CviRI cleaves DNA at TG/CA sequences. Nucleic Acids Res. 1994 Sep 25;22(19):3928–3929. doi: 10.1093/nar/22.19.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetsky EYu, Drutsa V. L., Kovaleva I. E., Luzikov V. N., Uvarov VYu Effects of amino-terminus truncation in human cytochrome P450IID6 on its insertion into the endoplasmic reticulum membrane of Saccharomyces cerevisiae. FEBS Lett. 1993 Dec 20;336(1):87–89. doi: 10.1016/0014-5793(93)81615-7. [DOI] [PubMed] [Google Scholar]
- Lennard L., Gibson B. E., Nicole T., Lilleyman J. S. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1993 Nov;69(5):577–579. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Lin J. S., Scott E. P., Pui C. H., Evans W. E. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther. 1994 Jan;55(1):15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Miller D. R., Evans W. E. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 May 1;341(8853):1151–1151. doi: 10.1016/0140-6736(93)93168-z. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Romiti P., Santerini S., Giuliani L. S-methyltransferases in human intestine: differential distribution of the microsomal thiol methyltransferase and cytosolic thiopurine methyltransferase along the human bowel. Xenobiotica. 1993 Jun;23(6):671–679. doi: 10.3109/00498259309059404. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz J. D., Kauma S., Guzelian P. S. Identification of the fetal liver cytochrome CYP3A7 in human endometrium and placenta. J Clin Invest. 1993 Aug;92(2):1018–1024. doi: 10.1172/JCI116607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz E., Gummert J., Mohr F., Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 Feb 13;341(8842):436–436. doi: 10.1016/0140-6736(93)93028-y. [DOI] [PubMed] [Google Scholar]
- Szumlanski C. L., Honchel R., Scott M. C., Weinshilboum R. M. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992 Aug;2(4):148–159. [PubMed] [Google Scholar]
- Tay B. S., Lilley R. M., Murray A. W., Atkinson M. R. Inhibition of phosphoribosyl pyrophosphate amidotransferase from Ehrlich ascites-tumour cells by thiopurine nucleotides. Biochem Pharmacol. 1969 Apr;18(4):936–938. doi: 10.1016/0006-2952(69)90069-0. [DOI] [PubMed] [Google Scholar]
- Van Loon J. A., Weinshilboum R. M. Thiopurine methyltransferase biochemical genetics: human lymphocyte activity. Biochem Genet. 1982 Aug;20(7-8):637–658. doi: 10.1007/BF00483962. [DOI] [PubMed] [Google Scholar]
- Vogt M. H., Stet E. H., De Abreu R. A., Bökkerink J. P., Lambooy L. H., Trijbels F. J. The importance of methylthio-IMP for methylmercaptopurine ribonucleoside (Me-MPR) cytotoxicity in Molt F4 human malignant T-lymphoblasts. Biochim Biophys Acta. 1993 Apr 30;1181(2):189–194. doi: 10.1016/0925-4439(93)90110-m. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Pazmiño P. A. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 May 2;85(3):323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]