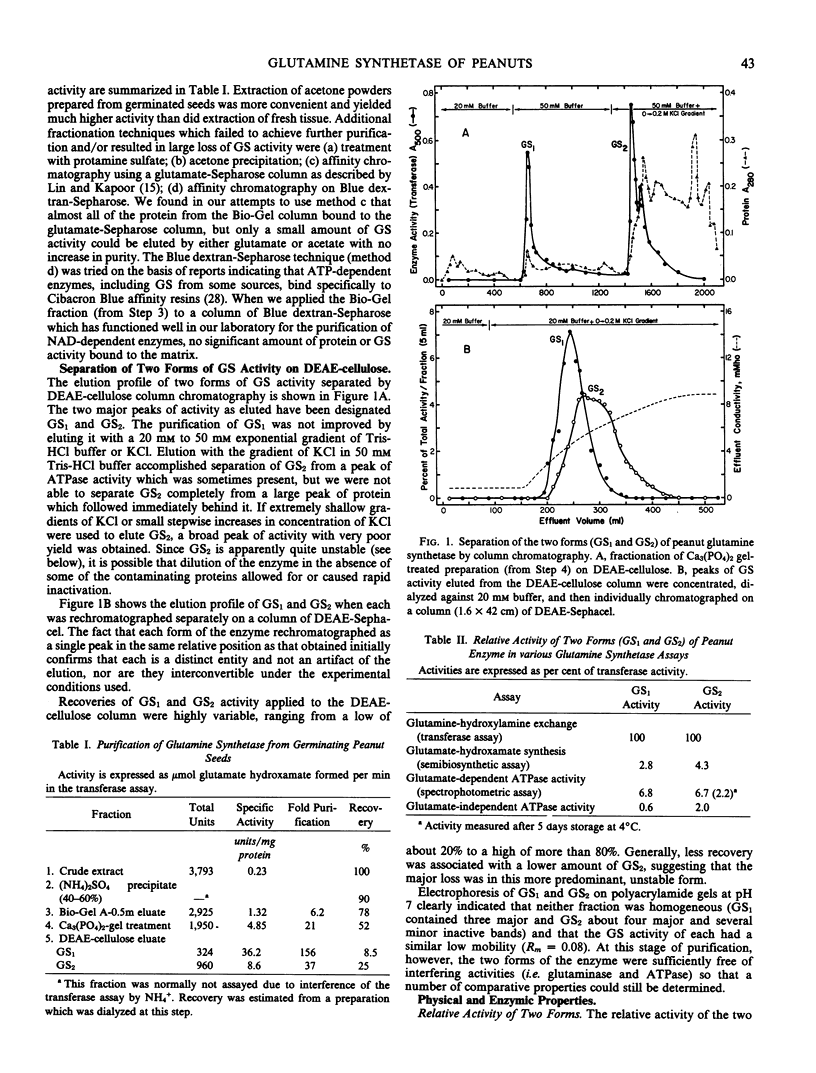
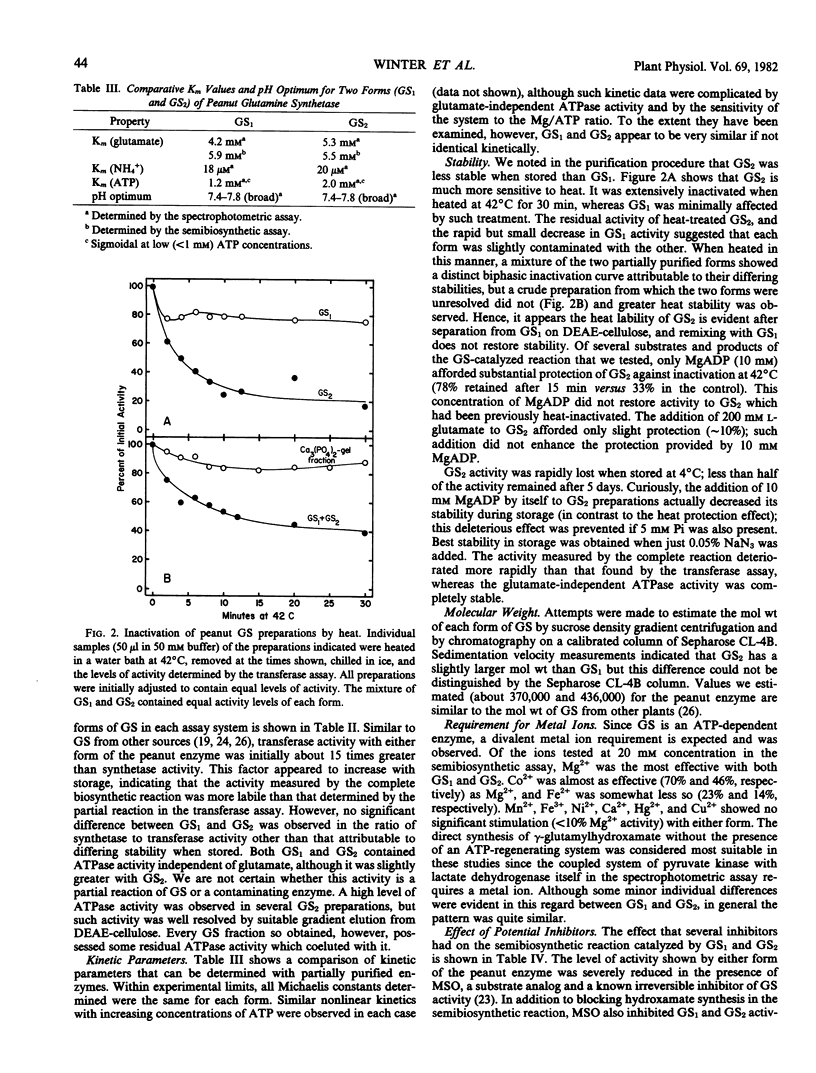
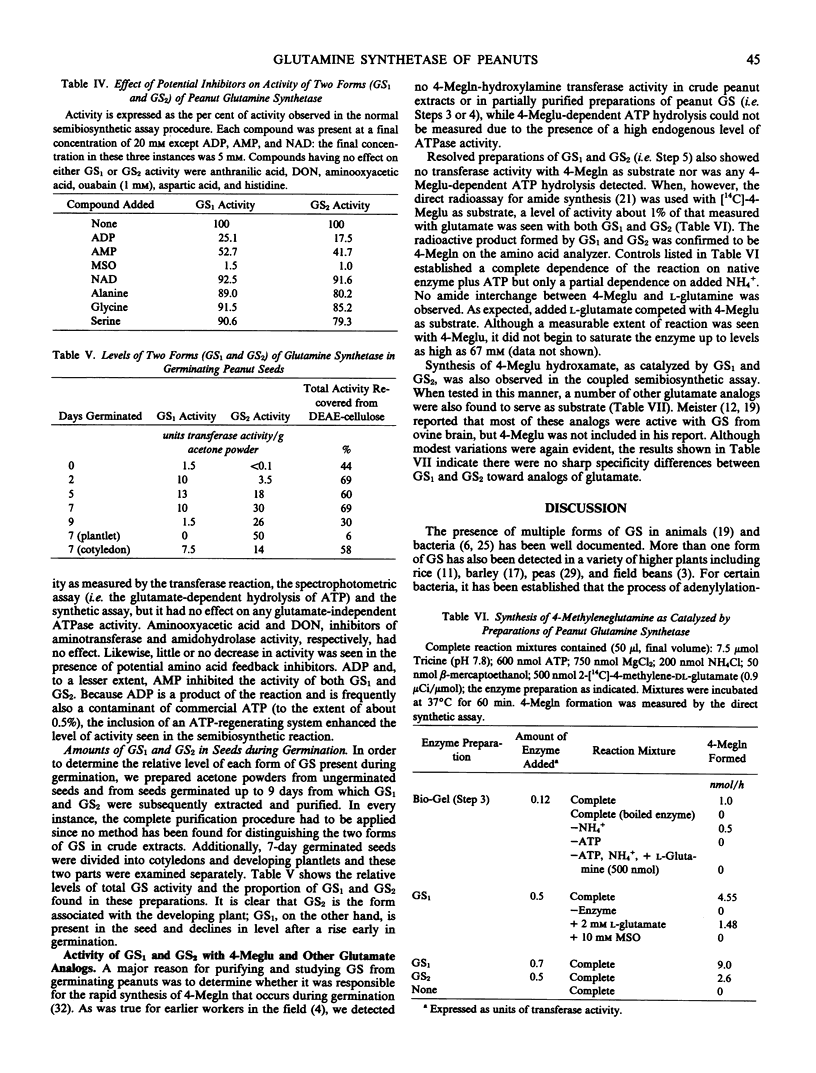
Abstract
Glutamine synthetase activity, extracted from an acetone powder of 7-day germinated peanuts (Arachis hypogaea L.), was precipitated by ammonium sulfate (40-60% saturation) and further purified by gel filtration and calcium phosphate gel treatment. When it was adsorbed to and subsequently eluted from a column of diethylaminoethyl-cellulose, two peaks of activity (designated glutamine synthetase 1 and 2) were obtained which were enriched 150- and 20-fold, respectively, over the initial extract. Glutamine synthetase 1 was present in ungerminated seeds and in the cotyledons during germination; glutamine synthetase 2 appeared during germination and was found largely in the developing plant. Compared with glutamine synthetase 2, glutamine synthetase 1 appeared to have a slightly smaller molecular weight and was more stable to heat and storage. The catalytic properties of the two forms were essentially the same. Whereas neither form catalyzed γ-glutamyltransferase activity with 4-methyleneglutamine, both glutamine synthetases 1 and 2 catalyzed an ATP- and NH4+-dependent conversion of [14C]-4-methyleneglutamic acid to [14C]-4-methyleneglutamine, but the Km value for 4-methyleneglutamic acid was 10-fold greater and the Vmax only one-fourth that measured with l-glutamic acid. This is the first report of glutamine synthetase activity with 4-methyleneglutamic acid as substrate, although the level of this activity does not appear adequate to account for the rapid synthesis of 4-methyleneglutamine observed in germinating peanuts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake J., Fowden L. Gamma-methyleneglutamic acid and related compounds from plants. Biochem J. 1964 Jul;92(1):136–142. doi: 10.1042/bj0920136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DEKKER E. E., MAITRA U. Conversion of gamma-hydroxyglutamate to glyoxylate and alanine; purification and properties of the enzyme system. J Biol Chem. 1962 Jul;237:2218–2227. [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Hirel B., Gadal P. Glutamine Synthetase in Rice: A COMPARATIVE STUDY OF THE ENZYMES FROM ROOTS AND LEAVES. Plant Physiol. 1980 Oct;66(4):619–623. doi: 10.1104/pp.66.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan H. M., Meister A. Activity of glutamine synthetase toward threo-gamma-methyl-L-glutamic acid and the isomers of gamma-hydroxyglumatic acid. Biochemistry. 1966 Jul;5(7):2423–2432. doi: 10.1021/bi00871a036. [DOI] [PubMed] [Google Scholar]
- Kingdon H. S., Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. XI. The nature and implications of a lag phase in the Escherichia coli glutamine synthetase reaction. Biochemistry. 1968 Jun;7(6):2136–2142. doi: 10.1021/bi00846a016. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L., MEISTER A. Reversibility of the enzymatic synthesis of glutamine. J Biol Chem. 1954 Jul;209(1):265–280. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. S., Kapoor M. Purification and studies of some physicochemical properties of glutamine synthetase of Neurospora crassa. Can J Biochem. 1978 Oct;56(10):927–933. doi: 10.1139/o78-144. [DOI] [PubMed] [Google Scholar]
- MARCUS A., FEELEY J., SHANNON L. M. Preparation and properties of gamma-methyleneglutamic acid. Arch Biochem Biophys. 1963 Jan;100:80–85. doi: 10.1016/0003-9861(63)90037-7. [DOI] [PubMed] [Google Scholar]
- Mann A. F., Fentem P. A., Stewart G. R. Identification of two forms of glutamine synthetase in barley (Hordeum vulgare). Biochem Biophys Res Commun. 1979 May 28;88(2):515–521. doi: 10.1016/0006-291x(79)92078-3. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- Pishak M. R., Phillips A. T. A modified radioisotopic assay for measuring glutamine synthetase activity in tissue extracts. Anal Biochem. 1979 Apr 1;94(1):82–88. doi: 10.1016/0003-2697(79)90793-0. [DOI] [PubMed] [Google Scholar]
- Powell G. K., Dekker E. E. A modified, high yield procedure for the synthesis of unlabeled and 14C-labeled 4-methylene-DL-glutamic acid. Prep Biochem. 1981;11(3):339–350. doi: 10.1080/00327488108061773. [DOI] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- SWINGLE S. M., TISELIUS A. Tricalcium phosphate as an adsorbent in the chromatography of proteins. Biochem J. 1951 Feb;48(2):171–174. doi: 10.1042/bj0480171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H. C., Powell G. K., Dekker E. E. 4-methyleneglutamine in peanut plants: dynamics of formation, levels, and turnover in relation to other free amino acids. Plant Physiol. 1981 Sep;68(3):588–593. doi: 10.1104/pp.68.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]



