Abstract
Objective:
Anabolic-androgenic steroids (AAS) are nominated for clinical use to promote protein synthesis in many therapeutic conditions. However, the indiscriminate use of AAS is related to hazardous cardiac disturbances and oxidative stress. We designed a study to investigate whether prolonged treatment with high doses of stanozolol modifies the activities of some antioxidant enzymes in the heart in sedentary and trained rats and whether this treatment causes alterations of cardiovascular parameters. In addition, the effectiveness of melatonin as an antioxidant and as a modulator of the cardiovascular side effects of stanozolol (STA) treatment was analyzed.
Materials and Methods:
Thirty male Wistar rats were divided into the following six groups: sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT). The stanozolol-treatment rats received 5 mg.kg−1 by subcutaneous injection before each exercise session (5 d.wk−1, i.e., 25 mg.kg−1.wk−1), while control groups received only saline solution injection. The melatonin-treatment groups received intraperitoneal injections of melatonin (10 mg.kg−1), 5 d.wk−1 for 6 wk. Electrocardiography, blood pressure and antioxidant enzyme activity measurements were performed at the end of the experimental period for cardiac function and molecular assessment.
Results:
This is the first time that the in vivo effects of melatonin treatment on stanozolol-induced cardiovascular side effects have been studied. Stanozolol induced bradycardia and significantly increased cardiac superoxide dismutase and catalase activities. Trained stanozolol-treated rats experienced an increase in blood pressure and relative heart weight, and they developed left cardiac axis deviation. Although melatonin did not prevent cardiac hypertrophy in exercised stanozolol-treated animals, it maintained blood pressure and cardiac catalase activity, and it prevented stanozolol-induced cardiac electrical axis deviation.
Conclusion:
In conclusion, under our experimental conditions, chronic stanozolol administration induced mild cardiovascular side effects that were partly attenuated by melatonin treatment. However, these results showed that the combination of melatonin and exercise could minimize the stanozolol side effects in the cardiovascular system.
Keywords: Stanozolol, melatonin, oxidative stress, anabolic effects, electrocardiogram, exercise
Özet
Amaç:
Anabolik-androjenik steroidler (AAS) bir çok tedavi koşullarında protein sentezini artırmak üzere klinik kullanıma aday gösterilmektedir. Ancak, AAS’in gelişigüzel kullanımı tehlikeli kalp bozuklukları ve oksidatif stres ile ilişkilendirilmiştir. Bu çalışma, sedanter ve antrenmanlı sıçanlarda stanozololun yüksek dozda uzun süreli kullanımının kalpte bazı antioksidan enzim faaliyetleri değiştirip değiştirmediğini ve bu tedavinin kardiyovasküler parametrelerde değişikliklere neden olup olmayacağını araştırmak için tasarlanmıştır. Buna ek olarak, bir antioksidan ve stanozolol (STA) tedavisinin kardiyovasküler yan etkilerinin bir modülatörü olan melatoninin etkinliği analiz edilmiştir.
Gereç ve Yöntem:
Otuz erkek Wistar sıçan aşağıdaki altı gruba ayrılmıştır; sedanter (S), stanozolol sedanter (SS), stanozolol-melatonin sedanter (SMS), antrenmanlı(T), stanozolol- antrenmanlı (ST) ve stanozolol-melatonin antrenmanlı (SMT). Stanozolol ile muamele edilen sıçanlar her egzersiz seansından önce cilt altı enjeksiyonu ile 5 mg.kg−1 (5 d.wk−1, yani 25 mg.kg−1.wk−1) stanozolol alırken, kontrol grubuna sadece serum fizyolojik enjeksiyonu yapılmıştır. Melatonin grupları için intraperitonal enjeksiyon ile 5 d.wk−1 (10 mg.kg−1) melatonin 6 hafta uygulanmıştır. Kalp fonksiyonu ve moleküler değerlendirilme amacıyla EKG, kan basıncı ve antioksidan enzim aktivite ölçümleri deney sürecinin sonunda yapılmıştır.
Bulgular:
Bu çalışma ile ilk defa stanozolola bağlı kardiyovasküler yan etkilerin melatonin ile tedavisinin in vivo etkileri incelenmiştir. Stanozolol bradikardiye neden olmuş ve önemli ölçüde kalp süperoksit dismutaz ve katalaz aktivitelerini arttırmıştır. Antrenmanlı olup stanozolol ile tedavi edilen sıçanlarda kan basıncı ve bağıl kalp ağırlığında bir artış gözlemlenmiş ve sol kalp aks sapması gelişmiştir. Melatonin uygulanması, stanozolol ile tedavi edilen hayvanlarda kalp hipertrofisine engel olamamasına rağmen, kan basıncı ve kalp katalaz aktivitesini muhafaza etmiş ve stanozolola bağlı kalp elektrik aks sapmasını engellemeyi başarmıştır.
Sonuç:
Sonuç olarak, deneysel koşullar altında, kronik stanozolol uygulanması, melatonin tedavisi ile kısmen zayıflatılmış olan hafif kardiyovasküler yan etkilere neden olmaktadır. Ancak, bu sonuçlar melatonin ve egzersiz kombinasyonunun kardiyovasküler sistemde stanozololun yan etkilerini en aza indirelileceğini göstermektedir.
Introduction
Stanozolol (STA) is a synthetic 17α-alkylated derivative of testosterone that exhibits a greater anabolic potency and a slower hepatic degradation than the natural male hormone [1]. While endogenous steroids are essential for the homeostatic functions of the body, exogenous steroids, i.e., anabolic-androgenic steroids (AAS), can be used to increase muscle mass, reduce body fat, and improve patient response to major trauma or surgery. AAS are used to improve muscular dystrophy, treat HIV patients, treat osteoporosis, alleviate symptoms of depression and anemia and reduce the effects of male hypogonadism [2, 3].
In addition to their therapeutic use, AAS are employed at suprapharmacologic doses in the context of sports to increase muscular development, physical performance, aerobic capacity and tolerance to high-intensity training [1]. The growing number of recreational athletes using ASS for aesthetic purposes makes this a public health concern [4]. Its indiscriminate use can substantially affect the cardiac system by increasing the risk of cardiovascular events, such as arrhythmias, high blood pressure (BP), changes in plasma lipid concentrations (increased total cholesterol and LDL, decreased HDL), reduced blood clotting time, polycythemia, cardiac ischemia, thrombosis, myocardial infarction, left ventricular hypertrophy and heart failure [3,5]. Moreover, many studies have shown a relationship between ASS abuse and disturbances in cardiac electrical impulse conduction, myocardial apoptosis and sudden death [6–9].
In recent decades, sound evidence has been generated to show that oxidative stress is one of the most potent inductors of endothelial dysfunction and cardiovascular disease. Contracting skeletal muscle, as well as cardiac muscle, generates increased amounts of reactive oxygen (ROS) and nitrogen species. ROS are byproducts of aerobic cellular metabolism, and antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) play crucial roles in circumventing their deleterious effects [5].
Under normal physiological conditions, the majority of ROS are produced in the mitochondrial electron transport chain during oxygen’s reduction to water in the mitochondria [10]. Because the prolonged administration of STA provokes dysfunction of mitochondrial respiratory chain complexes and mono-oxygenase systems, it is possible for these alterations to be accompanied by increased ROS generation [1, 11].
Melatonin (MEL), N-acetyl-5-methoxytryptamine, is the major hormone of the pineal gland, although it has also been detected in other tissues. It is a highly lipophilic molecule that crosses cell membranes easily to reach subcellular compartments, including mitochondria, where it seems to accumulate in high concentrations. Melatonin is able to prevent oxidative stress both through its free radical scavenging effect and by directly increasing antioxidant activity, and studies have demonstrated its protective role against oxidative damage induced by drugs, toxins, and different diseases [12].
Because no information is available on the effects of 17α-alkylated steroid treatment on cardiac antioxidant capacity, we designed a study to investigate whether a prolonged treatment with high doses of STA modifies the activities of anti-oxidant enzymes, such as catalase and superoxide dismutase, in the heart and whether this treatment causes alterations on cardiovascular parameters in sedentary and trained rats. MEL’s effectiveness as an antioxidant and modulator of cardiovascular side effects of STA treatment was also analyzed.
Materials and Methods
Training Protocol and Stanozolol and Melatonin Treatment
Thirty male Wistar rats (10 weeks old, weighing approximately 300 g) were obtained from the animal facilities of the State University of Campinas. They were housed in collective cages at 22–24°C on a 12-h light-and-dark cycle, with free access to tap water and food (standard chow for rodents - Purina).
Rat care, handling, and all of the experimental procedures employed were in accordance with the Ethics Committee on Animal Experimentation of Unicamp. After the adaptation period (7 d), animals were randomly distributed among the following 6 groups: sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) (n = 5 for each group).
The T, ST and SMT groups were submitted to the swimming protocol adapted from Radak et al. [13]. This protocol is an aerobic protocol without muscular damage, which could be induced by long-term running. The rats were submitted to swimming exercise, 5 d.wk−1 for 6 wk, in a water tank (85×56×60 cm and water temperature at 31±1ºC). Exercise sessions were initiated between 10:00 and 11:00 a.m. All of the rats were adapted to the water during the first week of the experiment. The adaptation process consisted of keeping the animals in shallow water, initially for 10 min and then progressively increasing 5 min/day and 5 cm water/day for 7 days. Therefore, the rats became accustomed to the depth and effort. This process led to reduced stress without promoting significant physical training adaptation. Exercise sessions began with 60 min/day during the second experimental week, and they were increased by 30 min each week until they reached 180 min of training during the sixth and last week of experiment.
The clinical human STA (Zambon, Barcelona, Spain) dosage is 0.5 mg.kg−1.wk−1. The animals received 5 mg.kg−1 STA by subcutaneous injection one hour before each exercise session (5 d.wk−1, i.e., 25 mg.kg1.wk−1); the CS and CT groups received only saline solution [3]. The high level of STA was chosen to simulate the massive doses of AAS used in sports.
The SMS and SMT rats received intraperitoneal injection of MEL (10 mg.kg−1), 5 d.wk−1 for 6 weeks. Injections were administered at 10:00 a.m. (approximately 30 minutes before exercise session), while the other groups received only saline [14].
After the experimental period, the rats were sacrificed by deepening the anesthesia. Hearts were excised to determine relative weight and then rapidly frozen in nitrogen and stored at −80ºC for further analysis.
Electrocardiogram (ECG)
ECG was performed on each rat before and after the experiment periods. Anesthetized rats (ketamine and xylazine, 90 mg/kg/bw and 45 mg/kg/bw, respectively, i.p.) were kept in the supine position with spontaneous breathing for ECG recording. The electrodes were connected to the computer channels (Heart Ware System), and six standard waves were recorded (I, II, III, aVR, aVR and aVF) with a sensitivity of 2N at a speed of 50 mm/second. The QT interval was measured in ten consecutive beats from the beginning of the QRS-complex to the point of return of the T wave to the isoelectric line, defined as the TP segment. QTd was calculated in absolute values by subtracting the shortest QT interval from the longest (QTd = QT max – Qt min). This value was converted into a percentage (QTd%) by correcting the QTd for the shortest QT interval and multiplying this value by 100 (QTd% = QT max – QT min/QT min x 100). The QT interval was corrected for the heart rate using Bazett’s formula (QTc = QT/√R-R), and the QTc interval dispersion was further calculated by subtracting the minimum QTc interval from the maximum QTc interval (QTcd = QTcmax - QTcmin). The percentage QTcd was also calculated (QTcd% = QTcmax - QTcmin/QTcmin x 100). The analyses were conducted by a single observer blinded to the treatment the animals received to minimize divergences in the dispersion measurement [15].
Blood Pressure
To determine arterial systolic and diastolic blood pressure, cannula were inserted into the left femoral artery and connected to BP-1-Analog single-channel transducer signal conditioner (World Precision Instruments, Sarasota, FL) [16].
Biochemical Assays
Heart samples were homogenized in an ice-cold isotonic 0.01 mol/L sodium phosphate buffer (pH 7.4) and centrifuged for 5 minutes at 12,000×g at 4°C. Catalase activity was examined in the supernatant by the spectrophotometric method described by Cohen et al. [17]. Briefly, the catalase-catalyzed decomposition of H2O2 was determined by subjecting the samples to reaction for 3 minutes with a standard excess of KMnO4 and subsequent measurement of the residual KMnO4 at 480 nm. Measurements were performed in triplicate. Protein concentrations were estimated by the bicinchoninic acid (BCA) method. Catalase activity was expressed as nM/µg protein content/min of the tissue homogenate for each group.
Superoxide dismutase activity was measured according to the method of Winterbourn et al. [18]. The principle of the assay was based on the ability of SOD to inhibit the reduction of nitro-blue tetrazolium (NBT). Briefly, the reaction mixture contained 2.7 mL of 0.067 M phosphate buffer (pH 7.8), 0.05 mL of 0.12 mM riboflavin, 0.1 mL of 1.5 mM NBT, 0.05 mL of 0.01 M methionine and 0.1 mL of enzyme samples. Uniform illumination of the tubes was ensured by an aluminum foil box under a 15 W fluorescent lamp for 10 min. A control without the enzyme source was included. The absorbance was measured at 560 nm. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction of NBT by 50% under the specific conditions. The SOD activity was expressed as nM/µg protein/ min of tissue homogenate compared to the control group.
Statistical Analysis
Statistical differences were calculated by analysis of variance (ANOVA) and Tukey’s test to establish differences between groups. To compare variations inside the group between the beginning and end of the experiment, we used Student’s t-test implemented in Prism software (Graphpad Software Inc., USA). The results are reported as means±SD, and differences were considered to be significant when p<0.05.
Results
Heart Rate (HR) and body weight (BW)
BW gain was reduced in both exercise groups (T and ST groups) when compared to the sedentary group (S). The STA-treated group had decreased body weight gain when associated with melatonin and/or exercise protocol (SMS and SMT versus SS and ST, respectively) (Figure 1). There was no significant difference in HR between experimental groups at the beginning of the experiment (showing that the groups were homogeneous). Although there was also no significant difference in HR between experimental groups at the end of the experiment, there was a statistically significant difference when compared within the group itself (between baseline and the end of experimental period). All groups except for the sedentary group exhibited a reduction in recovery HR (Table 1).
Figure 1.
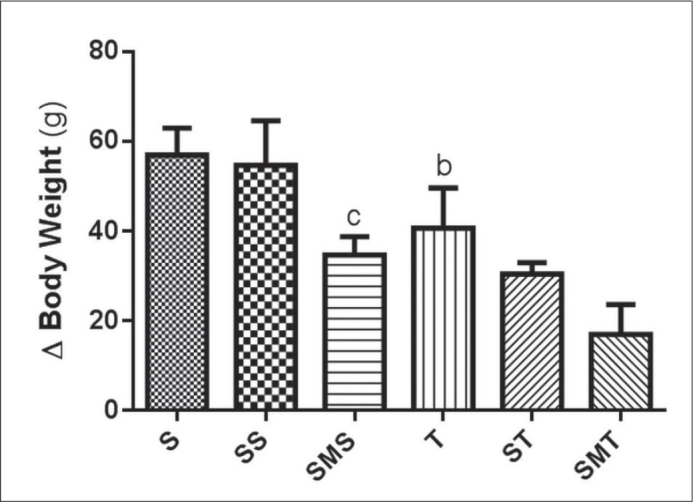
Effects of stanozolol treatment, melatonin treatment and/or exercise training on body weight variation of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups. The results are expressed as means±standard deviation; P <0.05 for (b) S × T, (c) SS × SMS.
Table 1.
Heart rates of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups, before the beginning of the experiment and after. The results are expressed as means±standard deviation.
| Group | Before | After |
|---|---|---|
| S | 458.6±7.4 | 449.8±10.0 |
| SS | 449.3±13.9 | 406.3±4.1* |
| SMS | 458.3±2.7 | 410.5±5.0* |
| T | 466.3±5.1 | 417.5±10.2* |
| ST | 467.8± 4.8 | 394.0±4.9* |
| SMT | 461.0±13.3 | 376.3±12.5* |
P<0.05, significantly different from pretreatment values.
Arterial Blood Pressure (BP)
Figure 2 shows the results of systolic and diastolic blood pressures for all experimental groups. When associated with exercise (ST group), STA increased systolic and diastolic arterial pressure; however, MEL administration maintained the BP (SMT group) similar to the control group.
Figure 2.
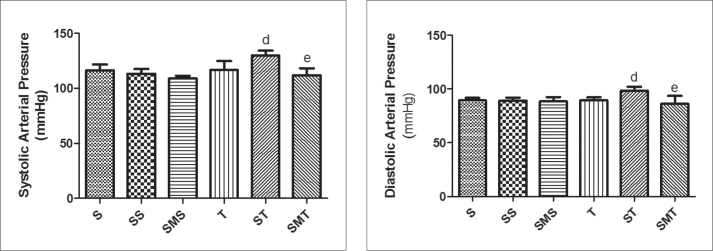
Systolic and diastolic arterial pressures of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups. The results are expressed as means ± standard deviation; P <0.05 for (d) T × ST; (e) ST × SMT.
Cardiovascular Parameters
Relative heart weight increased in the ST group when compared to the T group (Figure 3). MEL administration, despite preventing a raise in BP, did not prevent cardiac hypertrophy (SMT group). The ST group showed left cardiac electrical axis deviation (Table 2); however, the MEL-treated groups had no electrical axis deviation. There was no significant difference in other electrocardiographic parameters.
Figure 3.
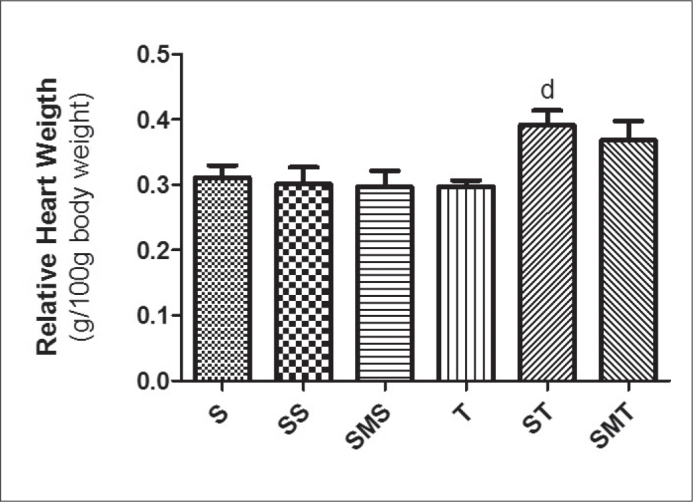
Relative heart weights of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups. The results are expressed as means±standard deviation; P <0.05 for (d) T × ST.
Table 2.
Cardiac axis deviations (º) of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups, before the beginning of the experiment and after. The results are expressed as means±standard deviation.
| Group | Before | After |
|---|---|---|
| S | 50.00±9.35 | 58.75±5.15 |
| SS | 60.00±5.00 | 56.67±7.26 |
| SMS | 52.50±4.79 | 56.00±3.08 |
| T | 54.17±2.71 | 60.33±1.33 |
| ST | 63.75±3.15 | 50.00±2.04* |
| SMT | 57.50±3.23 | 55.00±4.56 |
P<0.05, significantly different from pretreatment values.
Oxidative Stress Biomarkers
Figure 4 shows cardiac SOD and CAT activity values after the experimental protocol. Our results showed that all STA-treated groups had higher SOD activity than in the S group. Similarly, the trained groups showed increased heart SDO activity when compared to the control group. The cardiac catalase activity also increased in the STA-treated groups (SS and ST groups). MEL administration prevented the increase of cardiac CAT activity but had no effect on cardiac SOD activity.
Figure 4.
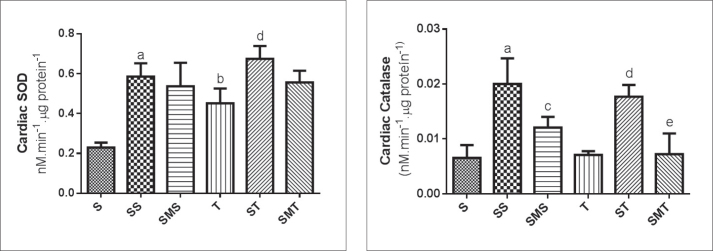
Cardiac SOD and CAT activities of Wistar rats in the sedentary (S), stanozolol sedentary (SS), stanozolol-melatonin sedentary (SMS), trained (T), stanozolol trained (ST) and stanozolol-melatonin trained (SMT) groups. The results are expressed as means ± standard deviation; P <0.05 for (a) S × SS; (b) S × T, (c) SS × SMS; (d) T × ST; (e) ST × SMT.
Discussion
Our study showed that prolonged (6 wk) MEL administration partly attenuated STA-induced cardiac side effects. This finding has great clinical relevance because AAS use (both clinical and ergogenic) has been increasing significantly over recent decades, and its side effects can be considered a public health concern [4]. Furthermore, our results showed that exercise combined with STA administration can have worse effects on cardiac tissue, and when associated with melatonin, these side effects can be minimized. This study was the first time that the in vivo effects of MEL treatment on STA-induced cardiovascular side effects have been studied.
STA treatment alone (SS group) did not change body weight, while MEL treatment decreased weight gain in sedentary rats treated with STA (SMS group). This result is in agreement with Wolden-Hanson et al. [19]. The authors demonstrated in rats that 12 weeks of MEL treatment without associated changes in food consumption decreased BW, relative intra-abdominal adiposity and plasma leptin and insulin levels while increasing spontaneous physical activity, body temperature and basal plasma corticosterone content toward youthful levels.
The verification of resting bradycardia in the exercise group (T group) demonstrated the effectiveness of the training protocol proposed in this study. Resting bradycardia has been considered to be the hallmark of the cardiovascular effect of exercise-training adaptation in both animals and humans [20].
Meanwhile, the STA treatment alone reduced the HR. This result is in agreement with the previous report of Beutel, Bergamaschi & Campos [3], who showed resting bradycardia in rats treated with STA. El-Mas et al. [21] showed that testosterone can act on the brainstem and preganglionic vagal cardiomotor neurons, modulating the autonomic cardiac control. Here, we also showed that STA promoted the increase of relative heart weight when combined with exercise, without changing the body weight gain. Our result is in agreement with Du Toit et al. [7], who also observed increased relative heart weight in rats submitted to exercise and AAS treatment. Many studies have shown that AAS may act on cardiomyocytes, leading to cardiac hypertrophy [3, 8]. Moreover, AAS abuse has been related to sometimes irreversible cardiomyocyte changes, such as concentric left ventricular hypertrophy [3, 6]. AAS anabolic effect is mainly mediated through androgenic receptors present in large amounts in skeletal and cardiac muscles. These androgenic receptors regulate transcription of target-genes promoting the accumulation of DNA required for muscle growth [22]. In our experiment, relative heart weight was significantly increased only in rats treated with STA and submitted to the exercise protocol (ST group). Both resistance training and long-term AAS exposure can enhance androgenic receptor content [23, 24]. This phenomenon would be a possible mechanism by which high doses of AAS, combined with exercise, could lead to cardiac hypertrophy [8]. Another factor of great importance regarding cardiac hypertrophy is collagen fiber content. Both STA and exercise can stimulate collagen synthesis in cardiac muscle, and the combination of these two factors could significantly increase the heart’s collagen content. Chronic AAS abuse, alone or combined with exercise, might promote pathological cardiac hypertrophy with cardiac function loss [7]. STA seems to stimulate collagen synthesis, due in large part to TGF- β1 (transforming growth factor- β1) action. This protein is a potent collagen stimulator that is normally secreted in the acute phase of inflammation and acts on tissue repair [2, 25].
STA promoted left axis deviation in the ST group; however, this deviation was within the normal range expected for rats. This result might be due to the increase in relative heart weight and cardiomyocyte area (data not shown). The mean cardiac axis represents the net direction and magnitude of cardiac electrical force. In essence, the QRS electrical axis is useful because it helps to determine the position of the heart in the chest, patency of electrical pathways and integrity of muscle mass. Despite some criticisms, a number of studies suggest that morphological aspect analysis of the electrical axis (amplitude and duration changes by ECG) is one of the primary tools to detect ventricular hypertrophy and sudden cardiac death [26]. QRS duration is frequently increased in left ventricular hypertrophy. The increased QRS duration might be attributed to the increased thickness of the left ventricle wall and to intramural fibrosis, which distorts and prolongs the transmural impulse propagation. Prolongation of the QT interval is indicative of potential risk of lethal arrhythmia and cardiac sudden death [27]. In our results, there was no difference in QRS, QT and QTc duration among experimental groups; hence, ventricular activation has not been impaired by STA treatment. Thus, the fact that duration of QT and QTc intervals have been presented with no changes among the experimental groups corroborates the values found for QRS, discarding the risk of sudden death (as verified in our experimental conditions) as suggested in previous studies as a consequence of AAS abuse [9, 28].
The lack of hypotension response in the exercise group is consistent with Medeiros [20], who reported no significant BP decrease in normotensive rats submitted to swimming training. In fact, exercise training has been recommended as a non-pharmacological treatment for high BP, but its effect in normotensive animals and humans seems to be minimal [29]. MEL was efficient for preventing BP elevation in exercised rats submitted to STA treatment (SMT group). MEL administration has been related to BP lowering due to several mechanisms, such as direct hypothalamic effect, catecholamine level reduction, smooth muscle relaxation and the antioxidant properties of MEL [30]. Spontaneously hypertensive rats submitted to MEL treatment experienced HR and BP reduction due to increased endothelium-dependent vasodilatation [31]. Moreover, BP decrease and baroreflex improvement in spontaneously hypertensive rats are related to long-term MEL treatment due to its antioxidant properties and decrease in the sympathetic drive [31]. Indeed, MEL-induced oxidative stress reduction might have contributed to HR and BP modulation, as we verified in the SMT group. MEL administration did not prevent cardiac hypertrophy in exercised animals treated with STA (SMT group); however, it prevented electrical axis deviation in this group. This result is in agreement with Simko et al. [31], who also observed that MEL did not reverse left ventricular hypertrophy in hypertensive rats, although fibrosis was reduced in hypertrophied ventricles (the authors also attributed this recution to its antioxidant properties, inhibiting the inflammatory process). It is known that NO and free radical availability might be involved in cardiac remodeling [30]. In a study with pinealectomized rats with a significant decrease in MEL secretion, Paulis & Simko [26] showed an increase in heart weight and cardiac fibrosis.
Another way to assess myocardial damage is to determine the effects of exercise and/or any treatment on the antioxidant system, which might be reflected by a high or low rate of ROS production during exercise in response to oxidative stress [27]. Intense and/or prolonged exercise sessions can cause oxidative stress in the heart; therefore, repetition of the exercise stimulus throughout the training period could activate the synthesis of antioxidant enzymes as a long-term strategy to cope with the oxidative stress encountered during exercise sessions [1]. The heart is equipped with all of the major antioxidant enzymes, i.e., SOD, CAT and glutathione peroxidase (GPx), as well as adequate levels of glutathione reductase (GRD) and glutathione S-transferases (GST). Regular physical exercise might beneficially influence cardiac antioxidant defenses and promote overall cardiac function. Several lines of evidence suggest that exercise induces adaptations in many tissues against an oxidative insult, mainly due to a mild oxidative stress, which could upregulate antioxidant enzyme gene expression through redox-regulated transcription factors [5]. In our experiment, swimming training and STA administration, alone or combined, increased cardiac SOD activity. MEL treatment did not prevent this enhanced activity. These results are in agreement with several studies showing that regular physical activity increases SOD activity in cardiac and skeletal muscle [5, 27]. On the other hand, Souza-Rabbo et al. [28] found decreased SOD activity in the heart after thirteen weeks of treadmill training in rats. According to Berra & Rizzo [29], MEL increases SOD activity, but this effect did not occur in our study. This different can be explained because, in our experiment, the rats were treated with MEL and also with STA, which is known to promote an increase in SOD activity. In addition, MEL also has direct anti-oxidant effects, which could lead to a decrease in ROS. This reduction in ROS generation could maintain or even reduce SOD activity.
Other studies have shown contradictory results related to the effect of exercise on the CAT enzyme. There are studies showing increase [30], no change [5], or decrease [27] in CAT activity after exercise. Exercise intensity and the moment that antioxidant enzyme activity analysis is performed after the last exercise session seem to be the key factors in revealing detectable effects of ROS on antioxidant enzyme activities in rodent hearts [30].
Training protocol alone did not change cardiac CAT activity, while STA induced elevation of cardiac CAT activity; this elevation was prevented by MEL administration. These results contradict those shown by Chaves et al. [5], who found no significant difference in cardiac CAT activity in rats treated with the AAS nandrolone decanoate when compared to the control group. In addition, Tomás-Zapico & Coto-Montes [31] showed an increase in CAT activity after treatment with MEL, while our study showed a decrease in CAT activity after treatment with MEL. We believe that the increase in CAT activity, or any other antioxidant enzymes, due to MEL-induced antioxidant enzyme activity does not occur in an environment where oxidative stress (and the changes induced by it) is already applied. Thus, the direct antioxidant effect of MEL would lead to decreased oxidative stress, consequently lowering antioxidant enzyme activity. Most of these studies did not use an exercise protocol, which obviously influences the responses of antioxidant enzyme activities.
In conclusion, the chronic use of supraphysiological doses of STA induced bradycardia and increased cardiac SOD and CAT activities. STA combined with swimming led to an increase in systolic and diastolic blood pressure, relative heart weight and left cardiac axis deviation. Although MEL did not prevent cardiac hypertrophy in exercised animals treated with STA, it prevented blood pressure elevation and the increase of cardiac catalase activity. MEL also prevented the STA-induced electric cardiac axis deviation. Hence, under our experimental conditions, the adverse effects of STA on the cardiovascular system were partly attenuated by MEL treatment. New experiments are now underway in our laboratory to investigate how MEL treatment could improve and minimize the side effects produced in the cardiovascular system. by STA administration.
Acknowledgments
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Pey A, Saborido A, Blázquez I, Delgado J, Megías A. Effects of prolonged stanozolol treatment on antioxidant enzymes activities, oxidative stress markers, and heat shock protein HSP72 levels in rat liver. J Steroid Biochem Mol Biol. 2003;87:269–77. doi: 10.1016/j.jsbmb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Falanga V, Greenberg AS, Zhou L, et al. Stimulation of collagen synthesis by the anabolic steroid stanozolol. J Invest Dermatol. 1998;111:1193–7. doi: 10.1046/j.1523-1747.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 3.Beutel A, Bergamaschi CT, Campos RR. Effects of chronic anabolic steroid treatment on tonic and reflex cardiovascular control in males rats. J Steroid Biochem Mol Biol. 2005;93:43–8. doi: 10.1016/j.jsbmb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: A looming public health concern? 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaves EA, Pereira-Junior PP, Fortunato RS, et al. JH. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol. 2006;99:223–30. doi: 10.1016/j.jsbmb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Urhausen A, Albers T, Kindermann W. Are the cardiac effects of anabolic steroid abuse in strength athletes reversible? Heart. 2004;90:496–501. doi: 10.1136/hrt.2003.015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Toit EF, Rossouw E, Van Rooyen J, Lochner A. Proposed mechanisms for the anabolic steroid-induced increase in myocardial susceptibility to ischaemia/reperfusion injury. Cardiovasc J S Afr. 2005;16:21–8. [PubMed] [Google Scholar]
- 8.Payne JR, Kotwinski PJ, Montgomery HE. Cardiac effects of anabolic steroids. Heart. 2004;90:473–5. doi: 10.1136/hrt.2003.025783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fineschi V, Riezzo I, Centini F, et al. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic findings in two fatal cases of bodybuilders. Int J Legal Med. 2007;121:48–53. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 10.Ji LL. Antioxidants and Oxidative Stress in Exercise. Soc Exper Biol Med. 1999;222:283–291. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 11.Pansarasa O, D’Antona G, Gualea MR, Marzani B, Pellegrino MA, Marzatico F. “Oxidative Stress”: effects of mild endurance training and testosterone treatment on rat gastrocnemius muscle. Eur J Appl Physiol. 2002;87:550–5. doi: 10.1007/s00421-002-0668-3. [DOI] [PubMed] [Google Scholar]
- 12.Molpeceres V, Mauriz JL, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. J Gerontol A Biol Sci Med Sci. 2007;7:687–95. doi: 10.1093/gerona/62.7.687. [DOI] [PubMed] [Google Scholar]
- 13.Radak Z, Toldy A, Szabo Z, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–92. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Acikel M, Buyukokuroglu ME, Aksoy H, Erdogan F, Erol MK. Protective effects of melatonin against myocardial injury induced by isoproterenol in rats. J Pineal Res. 2003;35:75–9. doi: 10.1034/j.1600-079x.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 15.Costa ECS, Gonçalves AA, Areas MA, Morgabel RG. Effects of metformin on QT and QTc interval dispersion of diabetic rats. Arq Bras Cardiol. 2008;90:232–8. doi: 10.1590/s0066-782x2008000400004. [DOI] [PubMed] [Google Scholar]
- 16.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J PhysiolRenal Physiol. 2006;291:495–502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 17.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 18.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cells superoxide dismutase activity. J Lab Clin Med. 1975;85:337–41. [PubMed] [Google Scholar]
- 19.Wolden-Hanson T, Mitton DR, McCants RL, et al. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–97. doi: 10.1210/endo.141.2.7311. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros A, Oliveira EM, Gianolla R, Casarini DE, Negrão CE, Brum PC. Swimming training increases cardiac vagal activity and induces cardiac hypertrophy in rats. Braz J Med Biol Res. 2004;37:1909–17. doi: 10.1590/s0100-879x2004001200018. [DOI] [PubMed] [Google Scholar]
- 21.El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol. 2001;38:754–63. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–11. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 23.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 24.The effects of exercise and training on human cardiovascular reflex control. J Auton Nerv Syst. 2000;81:16–24. doi: 10.1016/s0165-1838(00)00148-x. [DOI] [PubMed] [Google Scholar]
- 25.Paulis L, Šimko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol Res. 2007;56:671–84. doi: 10.33549/physiolres.931236. [DOI] [PubMed] [Google Scholar]
- 26.Simko F, Pechanova O, Pelouch V, et al. L. Effect of melatonin, captopril, spironolactone and simvastatin on blood pressure and left ventricular remodelling in spontaneously hypertensive rats. J Hypertens Suppl. 2009;27:5–10. doi: 10.1097/01.hjh.0000358830.95439.e8. [DOI] [PubMed] [Google Scholar]
- 27.Morán M, Delgado J, González B, Manso R, Megías A. Responses of rat myocardial antioxidant defences and heat shock protein HSP72 induced by 12 and 24-week treadmill training. Acta Physiol Scand. 2004;180:157–66. doi: 10.1111/j.0001-6772.2003.01244.x. [DOI] [PubMed] [Google Scholar]
- 28.Souza-Rabbo MP, Araújo AS, Fernandes TR, et al. Influence of exercise training frequency on cardiac and hepatic oxidative stress in rats. Exp Clin Cardiol. 2003;8:201–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Berra B, Rizzo AM. Melatonin: circandian rhythm regulator, chronobiotic, antioxidant and beyond. Clin Dermatol. 2009;27:202–9. doi: 10.1016/j.clindermatol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Ji LL. Handbook of Oxidants and Antioxidants in Exercise: exercise-induced oxidative stress in the heart. Elsevier Science B. V.; 2000. pp. 689–714. Part IX – Chapter 25. [Google Scholar]
- 31.Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x. [DOI] [PubMed] [Google Scholar]


