Abstract
Objective:
This study investigated the frequency of apoptosis in rat pulmonary epithelial cells after the injection of an intraperitoneal endotoxin lipopolysaccharide (LPS), the effects of LPS on apoptotic (bax, caspase-3) and antiapoptotic (bcl-2) markers during lung damage, and the protective effects of two known antioxidant agents, erdosteine and N-acetylcysteine (NAC).
Materials and Methods:
Male Wistar rats were divided into the following six groups, which included nine rats each: two control groups, two LPS-treated groups, one erdosteine-treated group (150 mg/kg), and one NAC-treated group (150 mg/kg). LPS was injected intraperitoneally at a dosage of 20 mg/kg. Following LPS injection, the antioxidants were orally administered. The rats were sacrificed at 24 h after LPS administration. The levels of apoptosis in bronchiolar and alveolar cells were determined using the TUNEL-staining method. Immunohistochemical staining of cytoplasmic bax, caspase-3, and bcl-2 in the epithelial cells was performed.
Results:
Erdosteine and NAC significantly reduced the rate of LPS-induced pulmonary epithelial cell apoptosis. The effect of NAC on regulating apoptosis was weaker than that of erdosteine. Erdosteine and NAC significantly reduced the local induction of bax and caspase 3 and significantly increased the reduced local production of bcl-2.
Conclusion:
These findings suggest that erdosteine and NΑC can effectively protect the lungs from the damaging effects of LPS.
Keywords: Apoptosis, epithelial cells, erdosteine, N-acetylcysteine, pathogenesis, sepsis
Özet
Amaç:
Bu çalışma, sıçanlarda intraperitoneal endotoksin (LPS) uygulanmasından sonra pulmoner epitel hücrelerdeki apopitozis sıklığını, akciğer hasarında apopitotik ve antiapopitotik göstergeler (bax, kaspaz-3 ve bcl-2) üzerine LPS’in etkilerini ve bilinen iki antioksidan ilacın (erdostein ve asetilsistein) koruyucu etkilerini araştırmak amacıyla yapıldı.
Gereç ve yöntem:
Sıçanlar, her biri 9 hayvandan oluşan 6 gruba ayrıldı. Bunlar; 2 negatif kontrol, 2 pozitif kontrol, erdostein (150mg/kg) ile tedavi edilen ve asetilsistein (150mg/kg) ile tedavi edilen gruplardı. Sıçanlara 20mg/kg vücut ağırlığı dozunda intraperitoneal olarak LPS solüsyonu enjekte edildi. LPS enjeksiyonunu takiben oral antioksidan ilaç tedavisine başlandı. Sıçanlar LPS uygulanmasından 24 saat sonra öldürüldü. Bronşiol ve alveol epitel hücrelerinde TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick endlabelling) yöntemi kullanılarak apopitozis düzeyi belirlendi. Epitel hücrelerindeki sitoplazmik bax, kaspaz-3 ve bcl-2 boyanması immünhistokimyasal olarak değerlendirildi.
Bulgular:
Erdostein ve asetilsistein tedavisi, sepsisin neden olduğu pulmoner epitel hücre apopitozisi oranını istatistiksel olarak önemli ölçüde azalttı. Asetilsisteinin epitel hücre apopitozis regülasyonu üzerine etkisinin erdosteine göre daha zayıf olduğu bulundu. Erdostein ve asetilsistein, lokal bax ve kaspaz-3 oluşum seviyelerindeki artışı istatistiksel olarak önemli ölçüde azalttı ve lokal bcl-2 seviyesindeki azalışı önemli ölçüde artırdı.
Sonuç:
Bu bulgular, Erdostein ve asetilsisteinin akciğerleri LPS’in zararlı etkilerinden korumada etkili olabileceğini göstermektedir.
Introduction
Sepsis caused by Gram-negative bacteria can lead to multiple organ dysfunction and exhibits a high mortality rate. The bacterial endotoxin lipopolysaccharide (LPS), which is found on the outer membrane of Gram-negative bacteria, is a key proinflammatory mediator that contributes, at least in part, to the deleterious effects of Gram-negative bacteremia and its associated endotoxemia [1]. Survival studies have provided the most compelling evidence that apoptotic changes in lymphoid tissues can contribute to the morbidity and mortality that is directly associated with sepsis [2]. During sepsis, the organ that is most susceptible to injury is the lung, and acute lung injury (ALI) leading to acute respiratory distress syndrome (ARDS) is a clinical syndrome that is associated with respiratory dysfunction that often complicates sepsis [3].
Apoptosis elicits a crucial function in maintaining tissue homoeostasis under physiological conditions and may also contribute to disease pathogenesis. Apoptosis can occur via the death receptor (extrinsic), mitochondrial (intrinsic), or endoplasmic reticular stress-induced pathways. These three pathways converge at the activation of cysteine-aspartate proteases (or “caspases”), which are triggered in response to proapoptotic stimuli, resulting in cellular disassembly [4].
The (extrinsic) death receptor pathway is triggered by signals from other cells and initiates when a death ligand, such as Fas or tissue necrosis factor (TNF), interacts with its cell surface receptors. The death receptor recruits adaptor proteins via its cytoplasmic domain, forming a death-inducing signal complex (DISC). The recruitment of caspase 8 to the DISC and its consequent activation result in the subsequent activation of effector caspases [5].
The (intrinsic) mitochondrial pathway involves the release of apoptogenic factors, such as cytochrome c (Cyt c) and apoptosis-inducing factor (AIF), from the mitochondria. Mitochondrial-dependent apoptosis is regulated by the bcl-2 family of proteins that includes both proapoptotic and anti-apoptotic molecules, the ratio between which partially determines the susceptibility of cells to the death signal [6]. The proapoptotic protein bax is located in the cytosol and is associated with intracellular membranes, including mitochondrial membranes. In response to mitochondrial-dependent stimuli, bax becomes activated and translocates to the mitochondrial outer membrane. After oligomerization, bax increases the permeability of the mitochondrial membrane, allowing for the release of Cyt c and other proapoptotic molecules from the intermitochondrial membrane space into the cytosol [7]. The interaction of Cyt c and ATP with the apoptotic protease activating factor-1 (apaf-I) in the cytosol facilitates the formation of the apoptosome complex [8]. Caspase 9 is recruited to this complex and becomes proteolytically activated, thereby initiating and amplifying effector caspase (e.g., caspase 3 and caspase 7) activation. Effector caspases are responsible for many of the biochemical characteristics of apoptosis, including the cleavage of the poly(ADP-ribose) polymerase (PARP) and, ultimately, DNA fragmentation. AIF is a conserved mitochondrial intermembrane flavoprotein that is released from the mitochondria and that translocate to the nucleus, where it subsequently plays an important role in the induction of nuclear chromatin condensation and DNA fragmentation [9].
The antiapoptotic protein bcl-2 is localized predominantly to the outer mitochondrial membrane and is tethered to the apaf-I complex. Bcl-2 preserves mitochondrial integrity and prevents the subsequent release of apoptogenic molecules, such as Cyt c [10].
In addition to the specific proteolytic cleavage events in the apoptotic signaling pathway, reactive oxygen species (ROS) are also generated during sepsis. It has been hypothesized that ROS act as upstream signaling molecules that initiate apoptosis [11, 12]. The regulation of apoptosis with agents known to augment the cellular antioxidant defense system and to neutralize ROS appears to represent a therapeutic tool for controlling the course of sepsis and the development of ALI.
Erdosteine [N-(carboxymethylthioacetyl)-homocysteine thiolactone] is a novel mucoactive agent that contains two blocked sulfhydryl groups, which are metabolized within the liver and can subsequently scavenge free radicals and act as an antioxidant [13]. N-acetylcysteine (NAC), the N-acetyl derivate of the amino acid l-cysteine, is a membrane-permeable aminothiol that reacts with reactive oxidative intermediates and replenishes the intracellular cysteine required for the production of glutathione (GSH), an endogenous antioxidant. NAC downregulates the activation of the transcription factor nuclear factor-κB (NF-κB), which is important in immune and inflammatory responses [14]. NAC is also an antiapoptotic molecule in several cell types [15].
This study investigated the frequency of apoptosis in rat pulmonary epithelial cells after the injection of an intraperitoneal (IP) endotoxin (LPS), the effects of LPS on apoptotic (bax, caspase-3) and antiapoptotic (bcl-2) markers in the context of lung damage, and the protective effects of two known antioxidant agents, erdosteine and NAC.
Materials and Methods
Animals
This study was conducted at the experimental research center, University of Akdeniz (Antalya, Turkey). Fifty-four Wistar rats (200–250 g) were used in the study. The animals were fed a commercial balanced diet and provided with tap water ad libitum. The rats were housed in cages and maintained at a controlled temperature (22±2°C) and level of humidity (55–60%) with a 12-h light/dark cycle. The investigation followed the National Research Council guidelines (NIH publication no. 85–23, revised 1985) and was approved by the Animal Care and Use Committee of the University of Akdeniz.
Experimental groups
The rats were divided into the following six groups, which included nine rats each: two negative control groups (IP saline plus oral distilled water or sodium bicarbonate), two LPS-treated groups (IP LPS plus oral distilled water or sodium bicarbonate), one erdosteine-treated group (IP LPS plus erdosteine at a dose of 150 mg/kg), and one NAC-treated group (IP LPS plus NAC at a dose of 150 mg/kg).
Experimental procedure
Lipopolysaccharide (LPS) from Escherichia coli 055:B5 (Sigma, St Louis, MO) was dissolved in 1 mL of sterile saline solution and injected intraperitoneally at a dosage of 20 mg/kg, as previously described [16]. Erdosteine (Sandoz Drug Industries; İstanbul, Turkey) was dissolved with an equivalent molar quantity of sodium bicarbonate in distilled water and NAC (Bılım Drug Industries; Istanbul, Turkey) was dissolved in distilled water. Following LPS injection, the antioxidants were administered orally as a single dose using a syringe with a gavage needle. The control rats were intraperitoneally administered isotonic saline solution at a volume equal to that of the LPS injection. Distilled water at a volume equal to that of the NΑC or a molar quantity of sodium bicarbonate equivalent to that of the erdosteine treatment was dissolved in distilled water and administered orally according to the drug administration protocol. The rats were sacrificed at 24 h after LPS administration by urethane anesthesia overdose, and a thoracotomy was performed for subsequent lung exploration. The lung tissue samples were processed for analysis of apoptosis, bax, caspase 3, and bcl-2.
Analysis of apoptosis
The level of apoptosis in the lung bronchiolar and alveolar epithelium was determined by using a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) kit (Roche; Mannheim, Germany), according to the manufacturer’s protocol. Briefly, the sections were deparaffinized and rehydrated. Next, the sections were incubated with proteinase K, were rinsed, were incubated in 3% H2O2, were permeabilized with 0.1% Triton X-100, were rinsed again, and were incubated in the TUNEL reaction mixture. Following incubation, the sections were rinsed and visualized using Converter-POD with DAB. The sections were counterstained with hematoxylin and eosin (H&E). Apoptotic cells containing DNA fragmentation at the single-cell level were identified by the TUNEL staining. The pulmonary epithelial cells per lung section were counted under a selected 400× microscopic field by two pathologists who were blinded to the experimental protocol. The apoptosis index was expressed as a percentage of TUNEL-positive cells in 1000 cells counted in the same section [17].
Analysis of bax, caspase 3, and bcl-2
The local production of bax and caspases 3 in the pulmonary epithelial cells was immunohistochemically evaluated using anti-bax (Abcam Ltd, Cambridge, UK) and anti-caspase 3 (NeoMarkers Inc. Portsmouth, NH, USΑ) kits, according to the manufacturers’ protocols. The local production of bcl-2 in the pulmonary epithelial cells was immunohistochemically evaluated using an anti-bcl-2 kit (Santa Cruz Group, Inc. USΑ), according to the manufacturer’s protocol. Briefly, the lung tissue samples on polylysine-coated slides were deparaffinized and rehydrated. Next, the microwave antigen retrieval procedure was performed, and the samples were incubated in a 3% H2O2 solution to inhibit endogenous peroxidases. To block nonspecific background staining, the sections were incubated with a blocking solution. Next, the sections were incubated with primary antibodies (anti-bax, anti-caspase 3, or anti-bcl-2), followed by incubation with a biotinylated goat anti-mouse antibody. After incubation with the chromogenic substrate (DAB), the sections were counterstained with hematoxylin and eosin (H&E). The slides were examined using a light microscope (Olympus BX51; Olympus Corp.; Tokyo, Japan) at 400×, and all of the analyses were performed by two pathologists who were blinded to the group assignments. Staining of cytoplasmic bax, caspase-3, and bcl-2 in pulmonary epithelial cells was evaluated (18–20). The results were expressed as the percentage of bronchial and alveolar epithelial cells that stained cytoplasmically positive in 1000 cells counted within the same section.
Statistical analysis
Statistical analyses were conducted using the SPSS statistical package (SPSS 9.0 for Windows, Chicago, IL, USΑ). The results were expressed as the mean values±standard deviation. Differences in quantitative variables between the groups were analyzed using a one-way analysis of variance (ANOVA) followed by post-hoc multiple comparison tests. All of the statistical tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
Results
Analysis of apoptosis
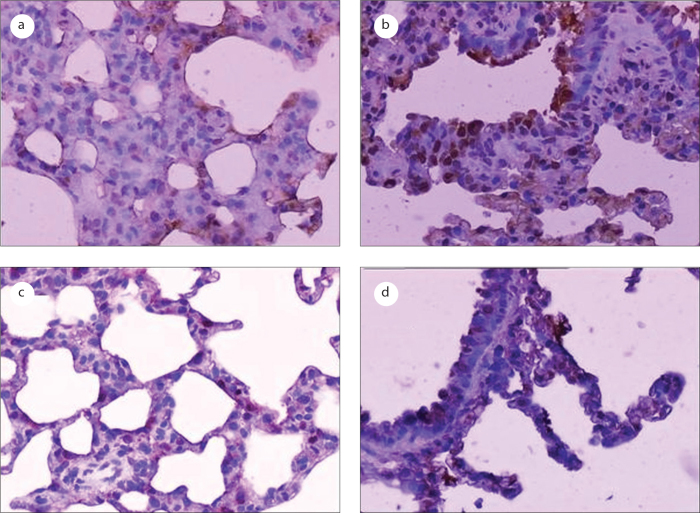
In this study, our analysis of apoptosis revealed no significant differences in apoptosis between the distilled water-treated group and the sodium bicarbonate-treated group (data not shown). The effects of the LPS and anti-oxidant treatments on apoptosis in the bronchiolar and alveolar epithelial cells are shown in Table 1. The numbers of TUNEL-positive bronchiolar and alveolar epithelial cells were significantly increased in the LPS-treated group compared to the control group (p=0). The administration of LPS resulted in the induction of apoptotic bodies, which are characteristic of apoptotic cell death (Figure 1). Erdosteine (p=0) and NAC (p=0) treatments significantly reduced the rate of LPS-induced pulmonary epithelial cell apoptosis. The effect of NAC on apoptosis in the bronchiolar and alveolar epithelial cells was weaker than that elicited by erdosteine (p=0.001).
Table 1.
The effects of the LPS and antioxidant treatments on apoptosis in the bronchiolar and alveolar epithelial cells
| Treated group | Apoptosis index (% cells staining positive) Mean±SD |
|---|---|
| IP saline | 10.6±8.5 |
| IP LPS | 85.6±6.2a |
| IP LPS + Erdosteine (150 mg/kg) | 19.4±9.8b |
| IP LPS + NAC (150 mg/kg) | 36.1±4.2b |
Significantly increased compared to the negative control group (p<0.0001).
Significantly reduced compared to the positive control group (p<0.0001).
Figure 1.
Photomicrographs of apoptotic cells (brown-stained nuclei) in the bronchiolar and alveolar epithelia as detected using the TUNEL method (original magnification, 200×). a: the negative control group; b: the LPS-treated group; c: the erdosteine-treated group; d: the NAC-treated group.
Analysis of bax
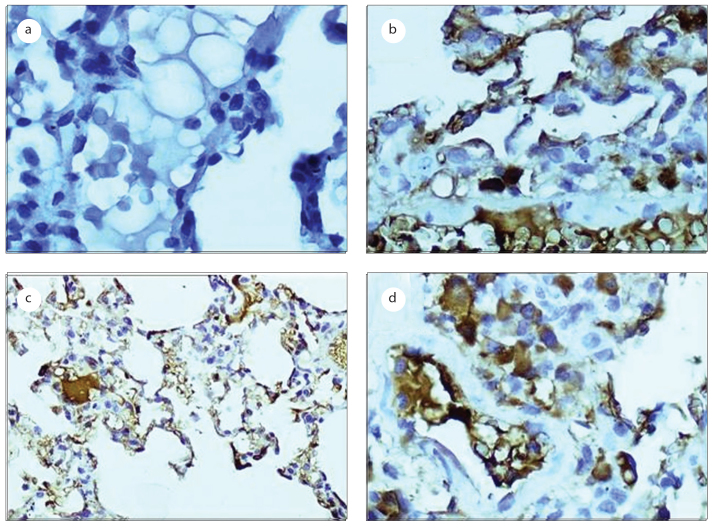
In this study, our analysis of bax revealed no significant differences in bax between the distilled water-treated group and the sodium bicarbonate-treated group (data not shown). The effects of the LPS and the antioxidants on the local production level of bax in the bronchiolar and alveolar epithelial cells are shown in Table 2. The percentages of bronchiolar and alveolar epithelial cells that stained cytoplasmically positive for bax were significantly increased in the LPS-treated group compared to that of the control group (p=0; Figure 2). Erdosteine (p=0) and NAC (p=0) treatments significantly reduced the increase in bax staining, and there were no significant differences detected in our analysis of bax between the two antioxidants groups (p=0.082).
Table 2.
The effects of the LPS and antioxidant treatments on the local production levels of bax, caspase 3, and bcl-2 in pulmonary epithelial cells
| Treated group | Local production level of bax (%) mean±SD | Local production level of caspase 3 (%) mean±SD | Local production level of bcl-2 (%) mean±SD |
|---|---|---|---|
| IP saline | 10.0±3.5 | 10.8±5.3 | 21.1±3.3 |
| IP LPS | 77.5±5.5a | 80.0±8.0a | 8.8±3.5c |
| IP LPS + Erdosteine (150 mg/kg) | 48.8±7.9b | 51.3±6.9b | 43.8±12.2d |
| IP LPS + NAC (150 mg/kg) | 2.8±2.6b | 43.9±3.3b | 45.6±5.3d |
Statistical analysis:
Significantly increased compared to the negative control group (p<0.0001).
Significantly reduced compared to the positive control group (p<0.0001).
Significantly reduced compared to the negative control group (p<0.0001).
Significantly increased compared to the positive control group (p<0.0001).
Figure 2.
Photomicrographs of the bax analysis in the bronchiolar and alveolar epithelial cells, as detected by immunohistochemical staining (original magnification, 400×). a: the negative control group; b: the LPS-treated group; c: the erdosteine-treated group; d: the NAC-treated group.
Analysis of caspase 3
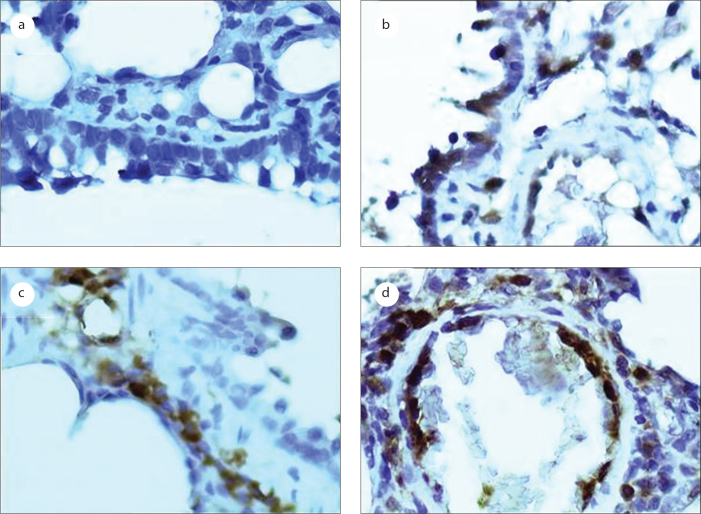
In this study, our analysis of caspase 3 revealed no significant differences in caspase 3 between the distilled water-treated group and the sodium bicarbonate-treated group (data not shown). The effects of the LPS and the anti-oxidants on the local production level of caspase 3 in the bronchiolar and alveolar epithelial cells are shown in Table 2. The percentages of bronchiolar and alveolar epithelial cells that stained cytoplasmically positive for caspase 3 were significantly reduced in the LPS-treated group compared the control group (p=0.005; Figure 3). Erdosteine (p=0) and NAC (p=0) treatments significantly reduced the increase in bax staining, and there were no significant differences detected in our analysis of bax between the two antioxidants groups (p=0.080).
Figure 3.
Photomicrographs of the caspase 3 analysis in the bronchiolar and alveolar epithelial cells, as detected by immunohistochemical staining (original magnification, 400×). a: the negative control group; b: the LPS-treated group; c: the erdosteine-treated group; d: the NAC-treated group.
Analysis of bcl-2
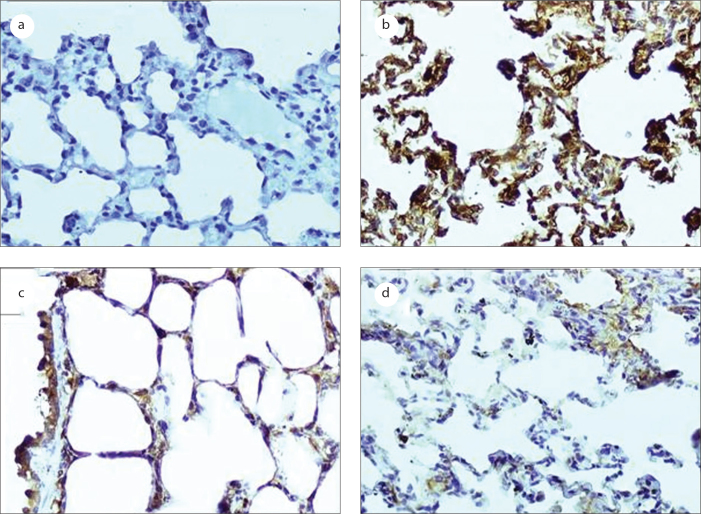
In this study, our analysis of bcl-2 revealed no significant differences in bcl-2 between the distilled water-treated group and the sodium bicarbonate-treated group (data not shown). The effects of the LPS and the antioxidants on the local production level of bcl-2 in the bronchiolar and alveolar epithelial cells are shown in Table 2. The percentages of bronchiolar and alveolar epithelial cells that stained cytoplasmically positive for bcl-2 were significantly reduced in the LPS-treated group compared to the control group (p=0.005; Figure 4). Post-treatment with erdosteine (p=0) and NAC (p=0) significantly increased the decrease in bcl-2 staining, and there were no significant differences detected in our analysis of bax between the two antioxidants groups (p=0.949).
Figure 4.
Photomicrographs of bcl-2 analysis in the bronchiolar and alveolar epithelial cells as detected by immunohistochemical staining (original magnification, 200×). a: negative control group; b: LPS-treated group; c: Erdosteine-treated group; d: NAC-treated group.
Discussion
In this study, the administration of LPS resulted in the induction of apoptotic bodies in bronchiolar and alveolar epithelial cells, which was indicative of the apoptosis of these cells. Treatment with erdosteine and NAC significantly reduced the rate of LPS-induced pulmonary epithelial cell apoptosis. The effect of NAC on regulating apoptosis in the epithelial cells was weaker than that elicited by erdosteine. These findings support the idea that apoptosis is a key element in the pathogenesis of sepsis and implicate possible pathways in this disease as future targets of therapeutic approaches [21]. Indeed, NAC has been shown to block both the death receptor and mitochondrial apoptotic pathways [15], whereas the effects elicited by erdosteine on the regulation of apoptosis have not been studied.
Mitochondria play a crucial role in apoptotic progression by releasing several apoptotic factors, such as Cyt c. The mitochondrial release of Cyt c represents a crucial step in the cellular apoptosis [6]. Although the mechanism underlying Cyt c release remains controversial, the involvement of the bcl-2 protein family in regulating Cyt c release is generally accepted [22]. The Bcl-2 protein is antiapoptotic and is localized within the mitochondria, endoplasmic reticulum, and nuclear membranes, where most ROS are generated to exert their apoptotic effects. Whereas Bcl-2 prevents the release of Cyt c into the cytosol after exposure to cytotoxic stimuli, proapoptotic bax induces the mitochondrial release of Cyt c, activating the caspase cascade and apoptosis [23].
In this study, the administration of LPS decreased the bcl-2 staining and increased the bax staining in the cytoplasm of epithelial cells, reflecting a marked reduction of the bcl-2/bax ratio that promotes cellular apoptosis. This indicates that bcl-2 and bax play important roles in sepsis-induced pulmonary epithelial cell apoptosis and that cell survival is associated with the ability of cells to maintain a homeostatic level of bcl-2 [24]. Treatment with erdosteine and NAC significantly reduced the increase in local production of bax and significantly increased the decreased local production of bcl-2, suggesting that antioxidants can prevent epithelial cell apoptosis. NAC inhibits the activation of c-Jun N-terminal kinase, p38 MAP kinase, redox-sensitive activator protein-1, and NF-κB transcription factor activities, which control the expression of specific antiapoptotic bcl-2 and inhibitors of apoptosis family proteins [25–27]. NAC can also prevent apoptosis and promote cell survival by activating the extracellular signal-regulated kinase (ERK) pathway [28].
Increasing evidence points to a significant role for bcl-2 and its family of cell-death regulating proteins in promoting both cell survival and cell death. Transgenic mice that overexpress the human bcl-2 gene within the lymphoid compartment not only exhibited reduced thymic and splenic apoptosis but also exhibited a survival advantage following sepsis. The anti-oxidant NAC and a combination of vitamins C and E have been shown to inhibit LPS-induced apoptosis, and the reduction in LPS-induced apoptosis by antioxidants has been shown to be paralleled by an increase in bcl-2 and a decrease in bax protein levels [24]. These results indicate that cell survival is associated with the ability of cells to maintain elevated levels of bcl-2.
Bcl-2 protects cells from the apoptosis that is induced by a variety of stimuli, but the underlying mechanisms are not completely understood. Bcl-2 preserves mitochondrial integrity and prevents the subsequent release of apoptogenic molecules such as Cyt c [29]. Several bcl-2 family members, such as bax, which are activated by diverse proapoptotic stimuli, translocate to the outer mitochondrial membrane, where they form homo-oligomers and a multimeric pore that facilitates the initial efflux of Cyt c [30]. The overexpression of bcl-2 reportedly confers protection of mitochondria, impairing the stimuli-induced opening of the permeability transition pore. Bcl-2 inhibits the intrinsic apoptotic pathway by preventing the activation of bax from disrupting the integrity of the outer mitochondrial membrane [15]. Furthermore, bcl-2 blocks bax translocation, possibly as a secondary consequence of its inhibition of bax activation [6].
Caspases are a family of proteolytic enzymes and play an essential role in the execution of apoptosis [3]. Individual members of the caspase family may be transiently activated and affect different stages of the signaling cascade that leads to apoptosis. During apoptosis, a proteolytic cascade, beginning with the activation of initiator caspases such as caspase 8, induces the activation of effector caspases such as caspase-3, which leads to the cleavage of several substrates and results in morphological changes observed in apoptosis [4]. The decisive role of caspases in the cell death program has been demonstrated by studies in which the pharmacological blockade of caspase activation was shown to prevent apoptosis and to improve organ function or survival in animal models of sepsis [20].
In this study, the administration of LPS increased the rate of caspase 3 staining in the cytoplasm of epithelial cells, suggesting that caspase 3 plays a central role in the sepsis-mediated apoptosis of pulmonary epithelial cells. Treatment of the cells with erdosteine or NAC significantly reduced the increase in caspase 3 staining. The activation of caspase 3 is widely accepted to represent the “point of no return” within the pathway of apoptotic death in mammalian cells [31]. Studies have found that the occurrence of cell apoptosis is closely associated with the mitochondrial release of Cyt c into the cytoplasm through permeability transition pores within the mitochondrial membrane and the activation of caspase 3 [32, 33]. NAC inhibits tonsillar B lymphocyte apoptosis. Its antiapoptotic activity is associated with its ability to inhibit the proteolytic processing of caspase-3 and caspase-7 and is accompanied by the inhibition of Cyt c release from mitochondria; it also increases the expression of bcl-2 and other survival proteins [25].
In conclusion, the results of this study suggest that the apoptotic and survival pathways are involved in the lung during the septic inflammatory response and that treatment with erdosteine and NAC can protect the lungs from the damaging effects of LPS. Therapeutic efforts at modulating the apoptotic response, particularly by interfering with the cell signaling pathways that lead to caspase-mediated apoptosis, represent an attractive therapeutic target for septic patients.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9:1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 2.Ayala A, Lomas JL, Grutkoski PS, Chung CS. Pathological aspects of apoptosis in severe sepsis and shock? Int J Biochem Cell Biol. 2003;35:7–15. doi: 10.1016/s1357-2725(02)00099-7. [DOI] [PubMed] [Google Scholar]
- 3.Czermak BJ, Breckwoldt M, Ravage ZB, et al. Mechanisms of enhanced lung injury during sepsis. Am J Pathol. 1999;154:1057–65. doi: 10.1016/S0002-9440(10)65358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayala A, Lomas JL, Grutkoski PS, Chung S. Fas-ligand mediated apoptosis in severe sepsis and shock. Scand J Infect Dis. 2003;35:593–600. doi: 10.1080/00365540310015656. [DOI] [PubMed] [Google Scholar]
- 5.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haraguchi M, Torii S, Matsuzawa SI, et al. Apoptotic protease activating factor 1 (Apaf-1)-independent cell death supression by Bcl-2. J Exp Med. 2000;191:1709–20. doi: 10.1084/jem.191.10.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes aand to trigger cytochrome c release from mitochondria. Biochem J. 2000;345:271–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Jürgensmeier J, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondira. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–96. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 10.Dlugosz PJ, Billen LP, Annis MG, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–96. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 12.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hypoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem. 2004;279:6753–60. doi: 10.1074/jbc.M310145200. [DOI] [PubMed] [Google Scholar]
- 13.Dechant KL, Noble S. Erdosteine. Drugs. 1996;52:875–81. doi: 10.2165/00003495-199652060-00009. [DOI] [PubMed] [Google Scholar]
- 14.Victor VM, Rocha M, De La Fuente M. N-acetylcysteine protects mice from lethal endotoxemia by regulating redox state of immune cells. Free Radic Res. 2003;37:919–29. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- 15.Jones DP, Maellaro E, Jiang S, Slater AF, Orrenius S. Effect of N-acetyl-L-cysteine on T-cell apoptosis are not mediated by increased cellular glutathione. Immunol Lett. 1995;45:205–9. doi: 10.1016/0165-2478(95)00004-o. [DOI] [PubMed] [Google Scholar]
- 16.Rudkowski JC, Barreiro E, Harfouche R, et al. Roles of iNOS and nNOS in sepsis-induced pulmonary apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:793–800. doi: 10.1152/ajplung.00266.2003. [DOI] [PubMed] [Google Scholar]
- 17.D’Αgostini F, Balansky RM, Izzotti Α, Lubet RA, Kelloff GJ, De Flora S. Modulation of apoptosis by cigarette smoke and cancer chemopreventive agents in the respiratory tract of rats. Carcinogenesis. 2001;22:375–80. doi: 10.1093/carcin/22.3.375. [DOI] [PubMed] [Google Scholar]
- 18.Krajewski S, Thor AD, Edgerton SM, Moore DH, 2nd, Krajewska M, Reed JC. Analysis of bax and bcl-2 expression in p53-immunopositive breast cancers. Clin Cancer Res. 1997;3:199–208. [PubMed] [Google Scholar]
- 19.Krajewska M, Wang HG, Karajewski S, et al. Immunohistochemical analysis of in vivo patterns of expression CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997;57:1605–13. [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–6. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Osman SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelarated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–8. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 22.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 23.Tan C, Dlugosz PJ, Peng J, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem. 2006;281:14764–75. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss R, Swanson PE, Knudson CM, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–56. [PubMed] [Google Scholar]
- 25.Rosati E, Sabatini R, Ayroldi E, et al. Apoptosis of human primary B lymphocytes is inhibited by N-acetyl-L-cysteine. J Leukoc Biol. 2004;76:152–61. doi: 10.1189/jlb.0403148. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Horie T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by pulmonary vascular endothelial cells. Br J Pharmacol. 2001;132:270–6. doi: 10.1038/sj.bjp.0703787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular Mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li WQ, Dehnade F, Zafarullah M. Thiol antioxidant N-acetylcysteine, activates extracellular signal-regulated kinase signaling pathway in articular chondrocytes. Biochem Biophys Res Commun. 2000;275:789–94. doi: 10.1006/bbrc.2000.3385. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–7. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 30.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem J. 2002;368:915–21. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthmann F, Wissel H, Schachtrup C, et al. Inhibition of TNF-alpha in vivo prevents hyperoxia-mediated activation of caspase 3 in type II cells. Respir Res. 2005;6:10. doi: 10.1186/1465-9921-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosulich SC, Savory PJ, Clarke PR. Bcl-2 regulates amplification of caspase activation by cytochrome c. Curr Biol. 1999;9:147–50. doi: 10.1016/s0960-9822(99)80068-2. [DOI] [PubMed] [Google Scholar]
- 33.Kirsch DG, Doseff A, Chau BN, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–61. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]