Abstract
Objective
There is currently substantial clinical interest in zinc (Zn) as an antioxidant and a protective agent against radiation-related normal tissue injury. To further assess the potential antioxidative effects, the effects of Zn were studied in rat lenses, a model of radiation-induced oxidative stress.
Materials and Methods
Sprague-Dawley rats were divided into three equal groups. Group 1 received neither Zn nor irradiation (control group). Group 2 (RT group) and 3 (RT+Zn group) were exposed to total cranium irradiation of 5 Gy in a single dose by using a cobalt-60 teletherapy unit. In addition to irradiation, group 3 was administered 10 mg/kg/day Zn. At the end of 10 days, the rats were killed. Their eyes were enucleated to measure the activities of antioxidant enzymes and the levels of iron, calcium, sodium and potassium.
Results
Irradiation significantly increased malondialdehyde levels as an end product of lipid peroxidation, glutathione peroxidase activity, and iron and calcium concentrations. Irradiation decreased super-oxide dismutase activities and zinc concentrations in the rat lens, indicating an increased oxidative stress generated by the decomposition of water and/or Fenton reaction. Malondialdehyde levels and iron and calcium concentrations were significantly decreased, and superoxide dismutase and glutathione peroxidase activities and zinc concentrations were increased, in the rat lenses of the RT+Zn group. No differences were detected in any final measurement of sodium and potassium in the direct comparison among all groups.
Conclusion
Zinc, acting as an antioxidant agent, may protect the lens from radiation-induced injury by improving oxidative stress generated by the decomposition of water and/or Fenton reaction.
Keywords: Radiotherapy, Oxidative stress, Zinc sulfate, Antioxidant enzymes, Trace elements
Özet
Amaç
Halen bir antioksidan ve radyasyona bağlı normal doku hasarına karşı koruyucu bir ajan olarak çinkoya (Zn) azımsanmayacak klinik ilgi vardır. Bu, ilacın potansiyel antioksidan etkilerini daha fazla değerlendirmek için, sıçanların lenslerinde radyasyona bağlı oluşan oksidatif stres modeli kullanılarak çinkonun antioksidan etkileri çalışıldı.
Gereç ve Yöntem
Sprague-Dawley türü sıçanlar üç eşit gruba bölündü. Grup 1 ne Zn ne de radyasyon aldı (kontrol grubu). Grup 2 (RT grubu) ve 3 (RT+Zn grubu) cobalt-60 teleterapi cihazı kullanılarak tek doz 5 Gy total kraniyal radyasyona maruz bırakıldı. Işınlamaya ek olarak grup 3’e 10mg/kg/gün Zn verildi. On günün sonunda sıçanlar sakrifiye edildi. Sıçanların gözlerine antioksidan enzimlerin aktiviteleri ve bazı elementlerin seviyelerinin ölçülmesi için enükleasyon yapıldı.
Bulgular
Işınlama anlamlı şekilde lipid peroksidasyonunun bir son ürünü olan malondialdehit seviyelerini, glutatyon peroksidaz aktivitesini, demir ve kalsiyum konsantrasyonlarını artırdı ve su ve/ veya fenton reaksiyonunun dekomposizyonu ile artmış oksidatif stresin göstergesi olarak sıçan lenslerinde çinko konsantrasyonu ve süperoksit dismutaz aktivitesi azaldı. Malondialdehit seviyelerinde, demir ve kalsiyum konsantrasyonlarında anlamlı bir azalma ve süperoksit dismutaz, glutatyon peroksidaz aktiviteleri ve çinko konsantrasyonlarında anlamlı artış, RT+Zn grubunda ki sıçanların lenslerinde bulundu. Tüm grupların direkt karşılaştırmasında sodyum ve potasyum son ölçümlerinde herhangi bir fark tespit edilmedi.
Sonuç
Çinko; su ve/veya fenton reaksiyonunun dekomposizyonu ile oluşan oksidatif stresin meydana getirdiği radyasyona bağlı hasardan bir antioksidan ajan olarak lensi koruyabilir.
Introduction
The killing action of ionizing radiation is predominantly due to ROS, including O2˙−, HO˙, and H2O2 generated by the decomposition of water and/or the Fenton reaction, which produces H2O2 and OH˙ via some metals such as iron (Fe+2), the most important Fenton reagent. The superoxide dismutase (SOD) enzyme catalyzes the dismutation of O2˙− into H2O2. H2O2 can be transformed into H2O and O2 by catalase (CAT) and glutathione peroxidase (GSH-Px) enzymes. One of the indices of oxidative damage is the malondialdehyde (MDA) formation as an end product of lipid peroxidation [1–4].
Ionizing radiation such as x-rays, gamma (γ)-rays and ultraviolet lights is known to be a cataractogenic factor for rat lenses [1,2]. Cataracts are an unavoidable complication if radiotherapy includes the orbit in the treated volume, even with very low doses of radiation [3]. Although the mechanisms of radiation-induced cataract formation are not clearly known, several mechanisms have been proposed. Because the damaging effects of ionizing radiation on living cells are predominantly due to reactive oxygen species (ROS), the first mechanism proposed for radiation-induced cataract is the theory of oxidative damage [1,2]. The second mechanism is DNA strand break induction within the lens epithelial cells and induction of apoptosis within the lens epithelial cell [5]. The third mechanism is a calcium (Ca+2) content increase in the lens [3], likely due to mitochondrial damage of lens epithelia similar to a chemical’s ocular toxicity potential [6,7]. All of these mechanisms may be related to the damaging effects of irradiation.
Over the past 30 years, numerous researchers have determined the pivotal role of zinc (Zn) in activities such as growth and development, maintenance and priming of the immune system, and tissue repair [8]. Many studies have shown Zn to be the catalytic component of more than 300 enzymes, the structural constituent of many proteins, and the regulatory ion for the stability of proteins and the prevention of free radical formation. Therefore, Zn is a pivotal element in assuring the functioning of various tissues and organs as well as the immune system [9].
Presently, there is no doubt that Zn as an essential trace element has an antioxidant role [10–12]. Two mechanisms have been elucidated: the protection of sulfhydryl groups against oxidation and the inhibition of the production of reactive oxygen molecules by transition metals [11,12]. Zn ions may induce the synthesis of metallothionein, sulfhydryl-rich proteins that are protective against the cytotoxic effects of reactive oxygen species, ionizing radiation, anti-cancer drugs and mutagens, and metals [9]. Zn is also the co-factor of copper-zinc superoxide dismutase (Cu-Zn SOD), an enzyme that acts as an important scavenger of superoxide radicals in the lens, thereby protecting against radiation-induced cataract formation [13]. There is currently substantial clinical interest in Zn as a protective agent against radiation-related normal tissue injury [14–18]. In order to further assess this compound’s potential antioxidative effects, the radioprotective effects of Zn were studied in rat lenses as a model of radiation-induced oxidative stress.
Materials and Methods
Rats and Experiments:
Thirty-six Sprague-Dawley rats, 10–12 weeks old, weighing 195 ± 25 g at the time of testing, bred at Atatürk University Medical School, Department of Pharmacology Experimental Animal Laboratory, were used for the experiment. All procedures involving Sprague-Dawley rats adhered to the ARVO Resolution on the Use of Animals in Research. The rats were quarantined for at least three days before irradiation, housed twelve to a cage in a windowless laboratory room with automatic temperature (22 ± 10C) and lighting controls (14 hr light / 10 hr dark), and fed standard laboratory feed and provided water ad libitum.
The rats were randomly divided into three equal groups. Group 1 did not receive Zn or irradiation (control group) but received 1 ml of saline orally plus sham-irradiation. Group 2 received total cranium gamma irradiation of 5 Gy as a single dose (RT group) plus 1 ml of saline orally. Group 3 received total cranium irradiation plus 10 mg/kg/day of Zn (RT+Zn group). Zinc sulfate (containing 50 mg zinc, Zinco 220 capsule, Berko, İstanbul) was administered to the RT+Zn group diluted in 1 ml of physiological saline through an orogastric tube beginning 3 days prior to irradiation and for 10 days after irradiation (13 days total). Excess oral ingestion of zinc to the point of toxicity (100 to 300 mg/ day) is rare. Zinc sulfate in amounts of 2 grams per day or more can cause gastrointestinal irritation and vomiting. In our study, 5 and 10 mg/kg/day doses of zinc sulfate were the calculated nontoxic doses for the gastrointestinal tract in a 70 kg (150 pound) of man. One milliliter of saline was administered daily through orogastric tube beginning 3 days prior to irradiation and for 10 days after irradiation (13 days total) to both the control group and the RT group.
Prior to total cranium radiotherapy, the rats were anesthetized with 50 mg/kg ketamine HCl (Pfizer, İstanbul, Turkey) and placed on a Plexiglas tray in a prone position. While the rats in the control group received sham-irradiation, the rats in the RT and the RT+Zn group were exposed to total cranium irradiation of 5 Gy in a single dose by using a cobalt-60 teletherapy unit (Picker, C 9, Maryland, NY, USA) with an output of 0.59 Gy/min to a 0.5 cm depth with a source-to-surface distance of 80 cm on a 5×5 cm anterior field. To maximize the dose delivered to the lens, a 0.5 cm thick wax bolus was placed on the rats’ eyes.
Fractionation of Samples:
At the end of 10 days, the rats were anesthetized with ether, and an intra-cardiac withdrawal of blood was performed. Following the withdrawal of blood, the rats were killed using a high dose of ether. Their eyes were enucleated, and the lenses were immediately dissected. The lenses were homogenized using an OMNI TH International, model TH 220 (Warrenton, VA, USA) homogenizer at the first speed setting. The right and left lenses of the rats were homogenized separately in isotonic saline and in deionized water (1/20 weight/volume) on ice for 10 seconds, respectively. The homogenate was centrifuged at 10,000 g for 60 min at 40 C. The supernatants were stored at −800 C in aliquots for biochemical measurements. Biochemical measurements for antioxidant status and trace metals were carried out on these supernatants spectrophotometrically.
Determination of MDA Levels:
Measurement of the MDA levels in the rat lenses was carried out using the method published by Ohkawa et al. [19]. Total thiobarbituric acid-reactive substances (TBARS) were expressed as MDA, using a molar extinction coefficient for MDA of 1.56 × 105 cm-1 M-1. MDA levels in the rat lenses were expressed as nmol/mg protein of lens sediment.
Determination of SOD Activity:
SOD activity in the rat lenses and erythrocytes was detected according to methods described by Sun and co-workers [20]. One SOD unit was defined as the enzyme amount causing 50% inhibition in the NBTH2 reduction rate. SOD activities in the rat lenses were expressed as U/mg protein of lens sediment.
Determination of GSH-Px Activity:
GSH-Px activity in the rat lenses was measured according to the Paglia and Valentina method [21]. By measuring the absorbance change per minute and by using the molar extinction coefficient of NADPH, GSH-Px activity of lens tissue was calculated. GSH-Px activities in the rat lenses were expressed as U/mg protein of lens sediment.
The protein content in the lens was determined using the Bradford method [22]. Biochemical measurements of antioxidant status were carried out at room temperature using a spectrophotometer (CECIL, CE 3041, Cambridge, UK). While biochemical measurements for zinc (Zn) and iron (Fe) were carried out at room temperature using an atomic absorption spectrophoto meter (Shimadzu AA-670, Kyoto, Japan), an automatic spectrophotometer (Olympus, AU-2700, Melville, NY, USA) was used for calcium (Ca), sodium (Na) and potassium (K).
Statistical Analyses:
The results were given as mean ± SD. Statistical analyses were performed using the SPSS packed program (Statistical Package for Social Science; Windows version 10.0) after the necessary data had been collected. Kruskal-Wallis non-parametric ana lysis of variance/orthogonal Mann Whitney tests were used to examine for contrasts among the treatment modalities. A p-value of less than 0.05 was accepted as statistically significant.
Results
The levels of MDA and the activities of SOD and the GSH-Px enzymes in the rat lenses are all presented in Figures 1, 2, and 3, respectively. In the RT group, the MDA level was higher than in the control group. There was a statistically significant difference between the RT group and the control group (P<0.05). In the RT+Zn group, the MDA level was lower than in the RT group. There was also a significant difference between the RT+Zn group and the RT group (P<0.05). There was no significant difference between the control group and the RT+Zn group. In the control group and the RT+Zn group, the SOD activity was higher than in the RT group, and this level was higher in the RT+Zn group relative to the control group. There was a significant difference between the RT group and the control group and the RT+Zn group (P<0.05). In the control group, the GSH-Px activity was lower than in the other groups. There was a statistically significant difference both in the RT group and the RT+Zn group when compared with those in the control group (P<0.05). In the RT+Zn group, the GSH-Px activity was higher than in the RT group, but there was not a significant difference between the two groups.
Fig. 1.
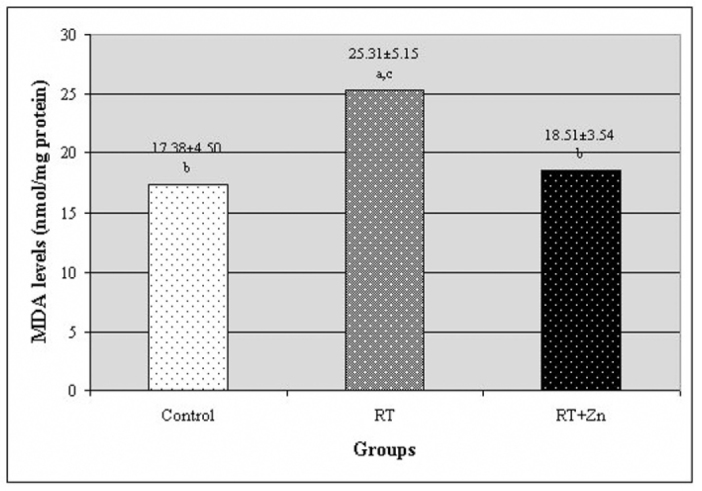
The level of malondialdehyde (MDA) in the rat lenses. Each data point (±SD) represents an average of twelve animals. (RT: irradiation, RT+Zn: irradiation plus zinc sulfate supplementation, a P<0.05 vs. control group, b P<0.05 vs. RT group, c P<0.05 vs. RT+Zn group).
Fig. 2.
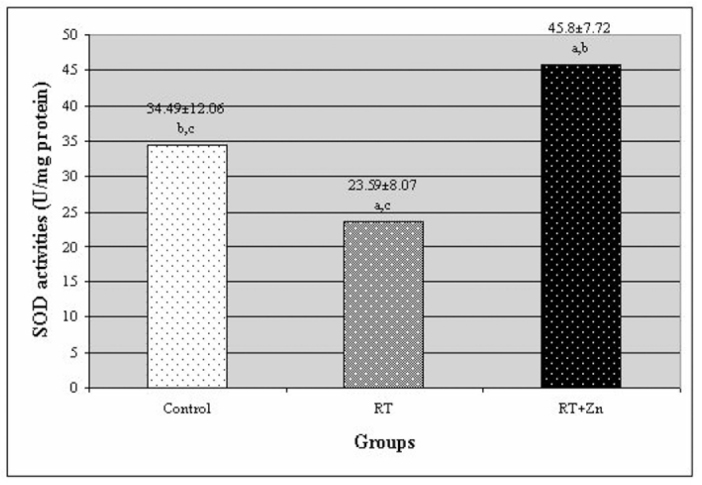
The activity of superoxide dismutase (SOD) enzyme in rat lenses. Each data point (±SD) represents an average of twelve animals. (RT: irradiation, RT+Zn: irradiation plus zinc sulfate supplementation, a P<0.05 vs. control group, b P<0.05 vs. RT group, c P<0.05 vs. RT+Zn group).
Fig. 3.
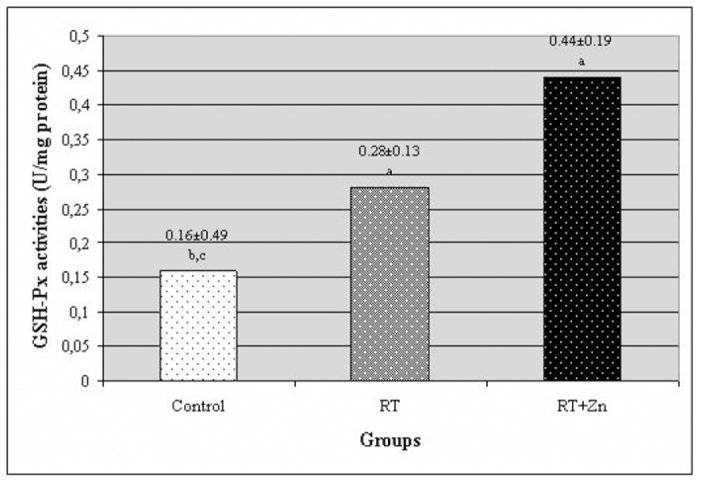
The activity of the glutathione peroxidase (GSH-Px) enzyme in rat lenses. Each data point (±SD) represents an average of twelve animals. (RT: irradiation, RT+Zn: irradiation plus zinc sulfate supplementation, a P<0.05 vs. control group, b P<0.05 vs. RT group, c P<0.05 vs. RT+Zn group).
The concentrations of Zn, Fe, Ca, Na and K in the rat lenses are all presented in Table 1. Fe and Ca concentrations in the lenses of the RT group were significantly higher, and Zn concentrations were significantly lower, compared with the lenses of the control and RT+Zn groups. There was not a statistically significant difference in the concentrations of Zn, Fe, Ca, Na and K between the control group and the RT+Zn group. No differences were detected in Na and K concentrations among all groups.
Table 1.
Concentrations of zinc (Zn), iron (Fe), calcium (Ca), sodium (Na) and potassium (K) in the rat lens. Each data point (±SD) represents an average of twelve animals.
| Groups | Zn μg/ml | Fe μg/ml | Ca mg/dl | Na nmol/L | K nmol/L |
|---|---|---|---|---|---|
| Control | 6.68 ± 0.46a | 14.18 ± 9.13a | 11.79 ± 4.88a | 16.33 ± 3.05 | 0.97 ± 0.40 |
| RT | 5.23 ± 0.2 b,c | 28.59 ± 0.64b,c | 19.47 ± 1.89b,c | 13.66 ± 0.57 | 0.52 ± 0.17 |
| RT+Zn | 6.55 ± 0.24a | 6.72 ± 2.01a | 6,91 ± 4.57a | 13,00 ± 1.00 | 0.90 ± 0.48 |
RT: irradiation, RT+Zn: irradiation plus zinc sulfate supplementation.
P<0.05 vs. control group,
P<0.05 vs. RT group,
P<0.05 vs. RT+Zn group
Discussion
The goal of radiation treatment is to deliver completely measured doses of ionizing radiation to a defined tumor volume while minimizing the injurious effects of ionizing radiation to the surrounding healthy tissue, thus eliminating tumor cells, giving a high quality of life and prolonging survival of cancer patients at reasonable cost [18]. When living cells are exposed to ionizing radiation, a variety of changes occur such as damage to cellular DNA and membrane structures and alterations in the immune system, depending on the exposed and absorbed dose, the duration of exposure and the interval after exposure, and the susceptibility of tissues to ionizing radiation [23]. Radiation is a known producer of ROS. When water, which constitutes approximately 80% of the cell, is exposed to ionizing radiation, decomposition occurs through which a variety of reactive oxygen species, such as the superoxide radical (O˙2̄), hydrogen peroxide (H2O2) and the hydroxyl radical (OH̄). The sensitivity of cells to ionizing radiation depends on the rate of differentiation as well as on the efficiency of the intrinsic antioxidative defense systems. Although all respiring cells are equipped with protective enzymes such as SOD and CAT or GSH-Px, increased oxidative stress in cells stemming from ionizing radiation may overwhelm the protective systems, leading to cell injury. The ROS formed in cells contribute to radiation injury in cells [3]. These ROS cause DNA strand breaks, lipid peroxidation and protein modification [4,24]. The ocular lens is one of the most radiosensitive tissues in the body, and the lens epithelium is considered to be the initiation site for the development of radiation-induced cataracts [24,25]. Because the damaging effects of ionizing radiation on living cells are predominantly due to ROS generated by the decomposition of water and/or the Fenton reaction [1,3,4], the theory of oxidative damage for cataract development is of interest [1,2].Bardak et al. [2] found that, one week after exposure, SOD and GSH-Px activities in the rat lens were lower in the group exposed to UVB than in the controls. In addition, the MDA level, which served as an index of cellular damage by free radicals, was higher in the UVB group than in the controls (P < 0.05). They suggested that the depletion of important intracellular antioxidant stores by UV-radiation in the lenses of the animals might have been the main cause of lens opacification. In a previous study, we determined that irradiation significantly increased both the MDA level and the activity of the GSH-Px, and significantly decreased the activity of the SOD in the rat lenses. This indicated the generation of oxidative stress and an early protective response to oxidative damage [26]. Avunduk et al. [1] demonstrated that 10 days after irradiation, Fe+2 and Ca+2 concentrations in the lens in the irradiated group were significantly higher, and that Zn+2 concentrations were significantly lower, compared with the control group. They proposed that enhancement of calcium influx in the presence of oxygen was related to accumulation of ROS that damages the membrane transport system of the lens. They also proposed that the iron-catalyzed Fenton reaction played an important role in x-ray-induced lens damage in the rat lens and that the decreased lens Zn+2 concentrations after irradiation indicated enhanced consumption of this antioxidant element and/or antioxidant enzymes as a counterbalance to the effects of oxidants.
In the present study, we found that the rat lens MDA level, an end product of lipid peroxidation, was significantly higher in the RT group than in the control group. We also found that the SOD activity in the RT group was significantly lower than in the control group, and that the GSH-Px activity in the RT group was significantly higher than in the control group. These results are consistent with previous studies. We demonstrated that, after irradiation, Fe+2 and Ca+2 concentrations in the rat lens in the RT group were significantly higher than in the control group. This is consistent with the hypothesis that the Fe+2-catalyzed Fenton reaction may play an important role in radiation-induced lens damage in the rat lens and that enhancement of Ca+2 influx in the presence of oxygen may be related to the accumulation of a ROS that damages the membrane transport system of the lens. We also demonstrated that, after irradiation, Zn+2 concentrations in the rat lens in the RT group were significantly lower than in the control group. This result is also consistent with the hypot hesis that the decreased Zn+2 concentrations in the rat lens may indicate increased consumption of this element subsequent to oxidative damage.
Zn has long been known as a trace element for living bodies [27]. Abundant evidence has demonstrated the antioxidant and anti-inflammatory roles of Zn in tissues, including the eye [11,12,27–29]. Zn plays a part in the maintenance of epithelial and tissue integrity by promoting cell growth and suppressing apoptosis and by protecting against free radical damage during inflammatory responses, an underappreciated role [30].
Currently, there is increasing evidence from human and experimental studies suggesting that Zn could be a beneficial agent in the protection against radiation-related normal tissue injury [14,18]. Zinc salts are a new class of radioprotectors against total body irradiation lethality [16]. In three recent studies, we de monstrated that zinc sulfate supplementation protected oropharyngeal mucosa against radiation-induced oropharyngeal mucositis in patients with head and neck cancer [14], white blood cells against radiation-induced hematopoietic toxicity in rats exposed to single-dose total body irradiation to 8 Gy [15] and the skin against radiation-induced dermatitis in a rat model [18]. Floersheim et al. [17] reported that Zn did not inhibit the radiotherapeutic effect of gamma rays on human tumors grown as xenografts in immunosuppressed mice and significantly reduced the fall in the hematocrit and numbers of thrombocytes, erythrocytes and leucocytes caused by irradiation, indicating a sparing effect on bone marrow precursors of peripheral blood cells. Ali et al. [31] reported that metallothionein induction by Zn was a highly effective approach in preventing cardiotoxicity and hepatotoxicity caused by daunorubicin in the rats. Based on the results of their experimental study, the authors suggested that the use of Zn in the chemotherapy of cancer patients was able to reduce daunorubicin-induced cardiotoxicity and hepatotoxicity. In addition, Provinciali et al. [32] reported that Zn exerted a direct selective action on cancer cells, inducing cell death through apoptosis. At the same time, Zn protects against apoptosis induced by diverse physical, chemical, or immunologic stimuli [33,34]. This means that zinc may not only protect intact tissues by decreasing apoptosis induced by injurious conditions but also increase apoptosis with a direct selective action on cancer cells. Briefly, Zn, as a radioprotective agent, may protect intact tissues against injurious effects of cancer treatments, such as chemotherapy or radiotherapy, without inhibiting the therapeutic effects.
Zinc, an essential element that affects cell metabolism by means of a variety of mechanisms, seems to play a pivotal role in maintaining normal ocular function. [27]. It has been demonstrated that irradiation decreased Zn concentrations in the rat lens [1]. Because of the antioxidant and anti-inflammatory effects of zinc [11,28], the decreased Zn concentrations interact with radiation damage in the lens. Because Zn is the co-factor of the Cu-Zn SOD enzyme that is an important scavenger of superoxide radicals in the lens, protecting against cataract formation due to irradiation [13], and because induction of DNA fragmentation under Zn-deficient conditions is a result of exchange of Zn for Ca and Mg within the nuclei with subsequent activation of endogenous endonucleases [33,35], these interactions may be related both to the insufficient activity of antioxidant enzymes such as SOD and GSH-Px and to the induction of DNA fragmentation under Zn deficient conditions. It is for these reasons that we tested zinc sulfate as a radioprotective agent in our study.
In the present study, we found that, in the RT group, the MDA level was significantly higher than in the RT+Zn group. We also found that the SOD activity was significantly higher in the RT+Zn group than in the RT group, and that the GSH-Px activity was higher in the RT+Zn group than in the RT group. However, this was not statistically significant. Because of these results, one can suggest that zinc sulfate supplementation might accelerate the activity of the lens SOD enzyme, and that the antioxidant system would be able to gradually clear away free radicals occurring in the environment as a result of Zn supplementation. We also found that Zn supplementation decreased the MDA level. We demonstrated that Zn supplementation increased the Zn concentration and decreased the Fe and Ca concentrations in the rat lens. According to these results, one may conclude that Zn supplementation protects the lens from radiation-induced cataracts via inhibition of the production of reactive oxygens by transition metals and prevents DNA fragmentation by inhibiting the exchange of Zn for Ca and Mg within the nuclei with subsequent activation of endogenous endonucleases.
Based on our study results, we suggest that Zn may protect against the damage produced by radiation by the up-regulation of antioxidant enzymes and by scavenging free radicals generated by ionizing radiation. These results should contribute to future studies that will examine the ability of zinc sulfate to limit radiation toxicity.
Footnotes
Conflict interest statement The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Avunduk AM, Yardimci S, Avunduk MC, Kurnaz L, Cengiz M. A possible mechanism of X-ray-induced injury in rat lens. Jpn J Ophthalmol. 2000;44:88–91. doi: 10.1016/s0021-5155(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 2.Bardak Y, Ozerturk Y, Ozguner F, Durmus M, Delibas N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr Eye Res. 2000;20:225–30. [PubMed] [Google Scholar]
- 3.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–93. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Choi IY, Kil IS, Kim SY, Yang ES, Park JW. Protective role of superoxide dismutases against ionizing radiation in yeast. Biochim Biophys Acta. 2001;1526:191–8. doi: 10.1016/s0304-4165(01)00126-x. [DOI] [PubMed] [Google Scholar]
- 5.Belkacemi Y, Piel G, Rat P, Julia F, Touboul E, Housset M. Ionizing radiation-induced death in bovine lens epithelial cells: mechanisms and influence of irradiation dose rate. Int J Cancer. 2000;90:138–44. [PubMed] [Google Scholar]
- 6.Bantseev V, McCanna D, Banh A, Wong W, Moran KL, Dixon DG. Mitochondria of lens epithelial and superficial cortical fibre cells as sensitive indicators of a chemical’s ocular toxicity potential (abstract) Invest Ophth Vis Sci. 2001;42:4729. [Google Scholar]
- 7.Kilic F, Trevithick JR. Modelling cortical cataractogenesis. XXIX. Calpain proteolysis of lens fodrin in cataract. Biochem Mol Biol Int. 1998;45:963–78. doi: 10.1002/iub.7510450514. [DOI] [PubMed] [Google Scholar]
- 8.Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 2001;79:170–7. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 9.Mocchegiani E, Muzzioli M, Giacconi R. Zinc metallothioneins, immune responses, survival and ageing. Biogerontology. 2000;1:133–43. doi: 10.1023/a:1010095930854. [DOI] [PubMed] [Google Scholar]
- 10.Bagchi D, Vuchetich PJ, Bagchi M, Tran MX, Krohn RL, Ray SD. Protective effects of zinc salts on TPA-induced hepatic and brain peroxidadion, glutathione depletion, DNA da mage and peritoneal macrophage activation in mice. Gen Pharmac. 1998;30:43–50. doi: 10.1016/s0306-3623(97)00072-4. [DOI] [PubMed] [Google Scholar]
- 11.Rotsan EF, DeBuys HV, Madey DL, Pinnell SR. Evidence supporting zinc as an important antioxidant for skin. Int J Dermatol. 2002;41:606–11. doi: 10.1046/j.1365-4362.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- 12.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281–91. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 13.Behndig A, Karlsson K, Reaume AG, Sent-man ML, Marklund SL. In vitro photochemical cataract in mice lacking copper-zinc superoxide dismutase. Free Radic Biol Med. 2001;31:738–44. doi: 10.1016/s0891-5849(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 14.Ertekin MV, Koc M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167–74. doi: 10.1016/s0360-3016(03)01562-1. [DOI] [PubMed] [Google Scholar]
- 15.Ertekin MV, Karslioglu I, Erdem F, Sezen O, Gepdiremen A, Serifoglu K. Zinc sulfate in the prevention of total body irradiation-induced early hematopoietic toxicity: Controlled a study in a rat model. Biol Trace Elem Res. 2004;100:63–73. doi: 10.1385/bter:100:1:063. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara J, Shida T, Ishioka K, Egawa S, Inada T, Machida K. Protective effect of zinc against lethality in irradiated mice. Environ Res. 1986;41:558–67. doi: 10.1016/s0013-9351(86)80150-5. [DOI] [PubMed] [Google Scholar]
- 17.Floersheim GL, Chiodetti N, Bieri A. Differential radioprotection of bone marrow and tumour cells by zinc aspartate. Br J Radiol. 1988;61:501–8. doi: 10.1259/0007-1285-61-726-501. [DOI] [PubMed] [Google Scholar]
- 18.Ertekin MV, Tekin SB, Erdogan F, Karslioglu I, Gepdiremen A, Sezen O. The effect of Zinc Sulphate in the prevention of radiation-induced dermatitis. J Radiat Res. 2004;45:543–8. doi: 10.1269/jrr.45.543. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res. 1978;19:1053–7. [PubMed] [Google Scholar]
- 20.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Karbownik M, Rieter RJ. Antioxidative effects of melatonin in protection againts cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 24.Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996;20:301–31. doi: 10.1016/0891-5849(96)02050-3. [DOI] [PubMed] [Google Scholar]
- 25.Baumstark-Khan C, Heilmann J, Rink H. Induction and repair of DNA strand breaks in bovine lens epithelial cells after high LET irradiation. Adv Space Res. 2003;31:1583–91. doi: 10.1016/s0273-1177(03)00095-4. [DOI] [PubMed] [Google Scholar]
- 26.Ertekin MV, Kocer I, Karslioglu I, Taysi S, Gepdiremen A, Sezen O. Effects of oral ginkgo biloba supplementation on cataract and oxidative stress occurring in lenses of the rat exposed to total cranium radiotherapy. Jpn J Ophthalmol. 2004;48:499–502. doi: 10.1007/s10384-004-0101-z. [DOI] [PubMed] [Google Scholar]
- 27.Grahn BH, Paterson PG, Gottschall-Pass KT, Zhang Z. Zinc and the eye. J Am Coll Nutr. 2001;20:106–18. doi: 10.1080/07315724.2001.10719022. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Mohamed G, Papapetropoulos A, Catravas JD, Caldwell RW. Zn2+ inhibits nitric oxide formation in response to lipopolysaccharides: implication in its anti-inflammatory activity. Eur J Pharmacol. 1998;341:265–72. doi: 10.1016/s0014-2999(97)01416-7. [DOI] [PubMed] [Google Scholar]
- 29.Tate DJ, Jr, Miceli MV, Newsome DA. Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radic Biol Med. 1999;26:704–13. doi: 10.1016/s0891-5849(98)00253-6. [DOI] [PubMed] [Google Scholar]
- 30.Berger A. Science Commentary: What does zinc do? BMJ. 2002;325:1062–3. [Google Scholar]
- 31.Ali MM, Frei E, Straub J, Breuer A, Wiessler M. Induction of metallothionein by zinc protects from daunorubicin toxicity in rats. Toxicology. 2002;179:85–93. doi: 10.1016/s0300-483x(02)00322-0. [DOI] [PubMed] [Google Scholar]
- 32.Provinciali M, Donnini A, Argentati K, Di Stasio G, Bartozzi B, Bernardini G. Reactive oxygen species modulate Zn(2+)-induced apoptosis in cancer cells. Free Radic Biol Med. 2002;32:431–45. doi: 10.1016/s0891-5849(01)00830-9. [DOI] [PubMed] [Google Scholar]
- 33.Sunderman FW., Jr The influence of zinc on apoptosis. Ann Clin Lab Sci. 1995;25:134–42. [PubMed] [Google Scholar]
- 34.Cabre M, Ferre N, Folch J, Paternain JL, Hernandez M, del Castillo D. Inhibition of hepatic cell nuclear DNA fragmentation by zinc in carbon tetrachloride-treated rats. J Hepatol. 1999;31:228–34. doi: 10.1016/s0168-8278(99)80218-9. [DOI] [PubMed] [Google Scholar]
- 35.McCabe MJ, Jiang S, Orrenius S. Chelation of intracellular zinc triggers apoptosis in mature thymocytes. Lab Invest. 1993;69:101–10. [PubMed] [Google Scholar]


