Abstract
Objective:
To compare the efficacy of intraarticularly injected bupivacaine with levobupivacaine when administered in combination with morphine and adrenaline for post-operative analgesia and functional recovery after knee surgery.
Materials and Methods:
Sixty American Society of Anesthesiologists physical status I–II patients were randomized into three groups: Group B was administered 30 mL isobaric 0.5% bupivacaine, 2 mg morphine and 100 μg adrenaline, Group L was administered 30 mL 0.5% levobupivacaine, 2 mg morphine and 100 μg adrenaline, and Group C was administered 30 mL 0.9% NaCl solution into the knee joint by the surgeon at the end of surgery. The morphine usage and visual analog pain scores were recorded regularly afterwards. We also recorded the time that elapsed before each patients’ first mobilization, positive response to straight leg raising, tolerance to 30–50° knee flexion, recovery of quadriceps reflexes and discharge from the hospital. We also recorded patient and surgeon satisfaction.
Results:
The pain scale values were lower in Groups B and L than in Group C at 2, 4, 6, 8, 12 and 24 hours post-operatively (all p<0.001). In Groups B and L, the time for first analgesic request was longer (p<0.01), the morphine consumption was lower (p<0.001), and the duration of morphine usage was shorter (p<0.001). The times to positive response to straight leg raising, tolerance to 30–50° knee flexion and the first mobilization were shorter in Groups B and L (p<0.001 for all).
Conclusion:
After arthroscopic knee surgery, intraarticular levobupivacaine combined with morphine and adrenaline decreases analgesic requirements, shortens the postoperative duration of analgesic use and hastens mobilization as effectively as bupivacaine.
Keywords: Arthroscopic surgery, bupivacaine, intraarticular injection, levobupivacaine, postoperative pain
Özet
Amaç:
Morfin ve epinefrin eklenerek intraartiküler uygulanan bupivakain ve levobupivakainin postoperatif ağrı ve fonksiyonel iyilşeme üzerine etkisini incelemek.
Gereç ve Yöntem:
ASA I–II 60 hasta randomize edilerek 3 gruba ayrıldılar: Grup B; 30 mL izobarik %0.5 bupivakain+2 mg morfin+100 mg adrenalin, Grup L; 30 mL %0.5 levobupivakain+2 mg morfin+100 mg adrenalin, Grup K; 30 mL serum fizyolojik cerrahi ekip tarafından operasyon bitiminde intraartiküler olarak uygulandı. Postoperatif morfin tüketimi, ağrı skorları, ilk mobilizasyon zamanları, 30–50 derece diz fleksiyonuna tolerans zamanları kaydedildi.
Bulgular:
Ağrı skorları Grup B ve L’de Grup K’ya göre 2, 4, 6, 8, 12 ve 24 satlerde daha düşük bulundu (p<0.001, tüm zamanlar için). Grup B ve L’de, ilk analjezik istem zamanı daha uzun (p<0.01), morfin tüketimi daha az (p<0.001) ve morfin kullanım süresi daha kısa bulundu (p<0.001). İlk mobilizasyon zamanları, 30–50 derece diz fleksiyonuna tolerans zamanları Grup B ve L’de daha kısa bulundu (p<0.001).
Sonuç:
Artroskopik diz cerrahsinde, intraartiküler levobupivakain, morfin ve adrenalin kombinasyonu bupibakaine benzer şekilde postoperatif analjezik gereksinimini, analjezik ihtiyaç süresini kısaltmakta ve mobilizasyonu iyileştirmektedir.
Introduction
Arthroscopic knee surgery is one of the most common surgical interventions. The examination of the knee joint of a patient with tuberculosis by Kenji Takagi with a cystoscope in 1918 was the first example of arthroscopy, which is now frequently used in outpatient surgery [1]. In the same year, Dr. Eugen Bircher published the first scientific article on diagnosis of meniscal pathology in the knee using an arthroscope [2]. Watanabe laid the foundations of arthroscopy with the improvements that he made to arthroscopes and other instruments [3].
Arthroscopy is relatively non-invasive and allows the patient to return to daily activities sooner [4]. Effective pain management is important after arthroscopic knee surgery to ensure that the patient is comfortable and can mobilize after discharge. An injection of local anesthetic to the intra-articular space, which is the main source of pain after arthroscopic interventions, has been found to provide effective and reliable results [5], as have morphine and non-steroidal anti-inflammatory drugs (NSAIDs) [6, 7]. However, the effects of combining bupivacaine or levobupivacaine with a mixture of morphine and adrenaline in an intra-articular injection at the end of arthroscopic knee surgery conducted under spinal anesthesia have not been studied. We conducted a randomized, controlled trial to examine the influence of both these drug combinations on post-operative morphine consumption, pain scores and functional recovery.
Materials and Methods
Sixty patients with American Society of Anesthesiologists physical status I–II between 18–70 years of age undergoing arthroscopic knee surgery were included in this prospective, randomized, double-blind study. We excluded patients taking NSAIDs, those with peptic ulcer disease, hemorrhagic diathesis or clotting dysfunction, allergy to local anesthetics and those who were pregnant, lactating or who declined to participate. All patients signed informed consent forms.
All participants received 50 mg intravenous dexketoprofen 30 minutes before surgery. In the operating room, routine monitoring was established, and an infusion of 0.9% NaCl (500 mL loading dose, then a 15 mL/kg/h maintenance dose) was started. Under aseptic conditions and after infiltration anesthesia with lidocaine at the L2–L3 or L3–L4 intervertebral space, spinal anesthesia was established by injecting 2 mL 0.5% hyperbaric bupivacaine (10 mg) through a 25G Spinocan® (B. Braun, Melsungen, Germany) needle after free flow of cerebrospinal fluid had been confirmed. The sensory block level and motor block were evaluated using pinprick and the Bromage scale (0: no paralysis, 1: can only move the knee and foot, 2: can only move the foot, 3: complete paralysis), respectively.
The subjects were divided into three equal groups using sealed envelopes. At the end of surgery and under aseptic conditions, Group B (n=20) received 30 mL intrathecal 0.5% bupivacaine with 2 mg morphine and 100 µg adrenaline; Group L (n=20) was administered 30 mL 0.5% levobupivacaine with 2 mg morphine and 100 µg adrenaline; and the control group (Group C, n=20) received 30 mL 0.9% NaCl solution.
Morphine sulfate was prepared in a 1 mg/mL concentration, and a post-operative patient-controlled analgesia (PCA) regime was established by providing a 2 mg bolus with a 10-minute lock-out time and a 4-hour limit of 20 mg. The severity of pain was evaluated with a visual analog scale (VAS, 0: no pain to 10: worst pain imaginable) at rest (VASR) and during movement (VASM) before surgery and 2, 4, 6, 8, 12 and 24 hours after surgery.
The heart rate (HR) and mean blood pressure (MAP) were recorded pre-operatively and 2, 4, 6, 8, 12 and 24 hours postoperatively. The time elapsed between the end of surgery and the first request for analgesia and total PCA consumption of morphine were recorded. The time to first mobilization, positive response to straight leg raising, tolerance of 30–50° knee flexion and return of the quadriceps reflex were recorded. The surgical time, patient and surgeon satisfaction ratings (bad, average, good), discharge time from hospital, requirement for follow-up consultations, intra- and post-operative complications (for example, bradycardia, hypotension, nausea and vomiting) were recorded, and the patients were asked if they would request the same mode of anesthesia again for a similar surgical procedure.
The power analysis was informed by the findings of a study by Karaman and colleagues [8]. When using the time to first analgesia as the primary outcome measure in groups of patients receiving intra-articular bupivacaine, levobupivacaine or placebo, the sample size required was calculated as 57 (19 for each group, power: 0.84, α=0.05, β=0.16).
The data were analyzed using SPSS 13.0 (Chicago, IL, USA). Continuous and discontinuous data are presented as the number and percent with the mean, standard deviation, median, minimum and maximum values. The distribution of data was examined using the Shapiro-Wilk test. The Kruskal-Wallis test was used to compare groups, and the Mann-Whitney U test was used for subgroup analysis. The chi-squared test was used to compare categorical variables between the groups. The extent of the change in pain was calculated using the pre- and post-operative VASR and VASM measurements before the groups were compared. The changes in the hemodynamic parameters were calculated from pre- and post-operative values, were expressed as percentages and were also compared between groups. A p<0.05 was considered to represent statistical significance.
Results
No patients declined to participate, and spinal anesthesia was successful in all cases. There were no statistically significant differences between the groups on the basis of their demographic characteristics (Table 1) or pre-operative HR, MAP, VASR and VASM scores. The post-operative VASR and VASM scores at 2, 4, 6, 8, 12 and 24 hours were significantly higher in Group C than in Groups B and L (p<0.001 for all, Tables 2 and 3).
Table 1.
Patient characteristics (mean±SD)
| Group B (n=20) | Group L (n=20) | Group C (n=20) | |
|---|---|---|---|
| Age (yr) | 37.5±13.0 | 41.8±12.7 | 41.4±11.6 |
| Gender (M/F) | 13/7 | 12/8 | 14/6 |
| Height (cm) | 169.5±8.8 | 169.2±12.1 | 169.5±7.6 |
| Weight (kg) | 71.5±11.7 | 76.4±13.2 | 76.2±14.6 |
| Duration of surgery (min) | 41.5±17.2 | 46.7±15.5 | 51.5±22.3 |
Table 2.
Post-operative pain scores at rest (VASR) (mean±SD)
| Post-operative hours
|
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 12 | 24 | |
| Group B (n=20) | 0 | 0.05±0.02 | 0.1±0.04 | 0.4±0.09 | 0.4±0.06 | 0.6±0.1 | 0.4±0.05 |
| Group L (n=20) | 0 | 0.05±0.03 | 0.2±0.03 | 0.3±0.07 | 0.4±0.07 | 0.4±0.08 | 0.2±0.04 |
| Group C (n=20) | 0 | 0.8±0.1* | 1.10±0.4* | 1.7±0.8* | 1.5±0.5* | 1.4±0.9* | 1.2±0.3* |
p<0.001; Group C vs Groups B and L.
Table 3.
Post-operative pain scores during mobilization (VASM) (mean±SD)
| Post-operative hours
|
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 12 | 24 | |
| Group B (n=20) | 0 | 0.2±0.08 | 0.7±0.2 | 1.4±0.3 | 1.8±0.5 | 1.4±0.4 | 0.8±0.1 |
| Group L (n=20) | 0 | 0.4±0.12 | 0.5±0.5 | 1.2±0.4 | 1.2±0.7 | 1.2±0.3 | 0.6±0.2 |
| Group C (n=20) | 0 | 1.5±0.7* | 2.7±1.1* | 3.3±1.3* | 3.4±1.8* | 3.1±1.2* | 2.9±1.1* |
p<0.001; Group C vs Groups B and L
The post-operative HR was significantly higher at 6 and 8 hours in Group C compared with Groups B and L (p<0.05 for both, Figure 1). There was no statistically significant difference in post-operative MAP between the three groups (Figure 2).
Figure 1.
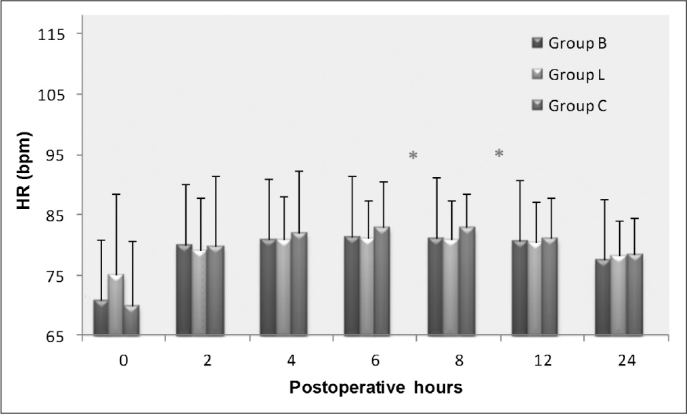
Postoperative changes in heart rate (HR[bpm]). *p<0.05; Group C vs Groups B and L.
Figure 2.
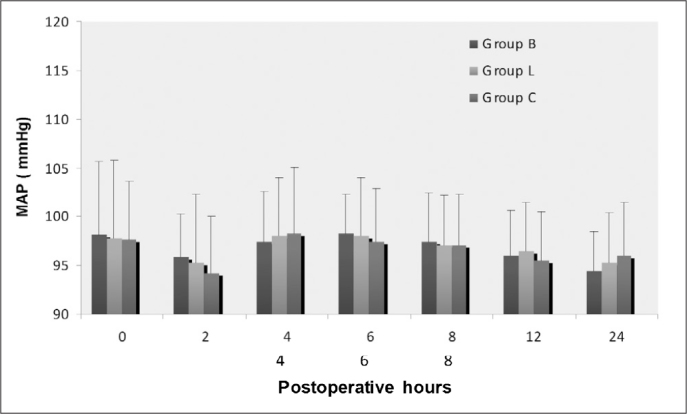
Postoperative changes in mean arterial pressure (MAP[mmHg]).
Analgesic consumption via PCA was similar in Groups B and L but was significantly higher at 6, 8, 12 and 24 hours (p<0.05, p<0.001, p<0.001 and p<0.001, respectively, Figure 3) in the control group. The time to first request for analgesia was also significantly shorter in the controls, who also used PCA for longer and consumed a higher total dose (all p<0.01, Table 4).
Figure 3.
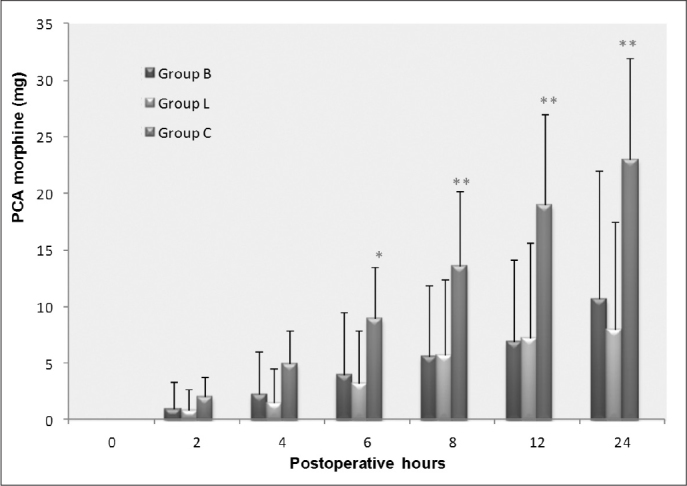
Cumulative intravenous morphine consumption delivered by patient-controlled analgesia (PCA) devices during a 24-hour study period. *p<0.05 or **p<0.001; Group C vs Groups B and L.
Table 4.
Post-operative clinical data (mean±SD)
| Group B (n=20) | Group L (n=20) | Group C (n=20) | |
|---|---|---|---|
| Time to first mobilization (min) | 256.25±19.6 | 280.75±15.31 | 314.25±15.58* |
| Time to straight leg raise (min) | 165.50±16.58 | 180.85±11.21 | 255.50±14.21* |
| Tolerance of 30–50° knee flexion (min) | 172.0±10.53 | 193.25±13.27 | 250.10±15.21* |
| Time to first analgesic requirement (h) | 258.5±19.8 | 251.5±17.4 | 223.2±17.8* |
| Time to first use of PCA (min) | 430±36.7 | 411±39.7 | 874.0±40.7* |
| Time to hospital discharge (h) | 19.15±4.5 | 20.15±3.37 | 21.90±3.92 |
p<0.01; Group C vs Groups B and L
PCA; patient-controlled analgesia
The time to first mobilization, positive response to the straight leg raising test and time to tolerance of 30–50° knee flexion were longer in the controls than in Groups B and L (p<0.001 for all). There were no significant differences in the duration of hospitalization. Patient and surgeon satisfaction scores were lower in Group C compared with the local anesthetic groups (p<0.01). There was no difference between the groups when patients were asked if they would have the same mode of anesthesia again (Table 5). No surgical, anesthesia-related, intra-operative or post-operative complications were observed.
Table 5.
Patient and surgeon satisfaction with pain management (n, %)
| Group B (n=20) | Group L (n=20) | Group C (n=20) | ||
|---|---|---|---|---|
| Patient satisfaction | Poor | 0 (0) | 0 (0) | 1 (5) |
| Moderate | 3 (15) | 1 (5) | 11 (55)* | |
| Good | 17 (85) | 19 (95) | 8 (40)* | |
| Surgeon satisfaction | Poor | 0 (0) | 0 (0) | 1 (5) |
| Moderate | 2 (10) | 2 (10) | 11 (55)* | |
| Good | 18 (90) | 18 (90) | 8 (40)* |
p<0.001; Group C vs Groups B and L
Discussion
In our study, the efficiency of bupivacaine or levobupivacaine addition to the morphine and adrenaline mixture applied to patients having elective arthroscopic knee surgery under spinal anesthesia on analgesia and functional resilience was compared. We found that patients who received intra-articular local anesthetic, morphine and adrenaline after arthroscopic knee surgery conducted under spinal anesthesia experienced less post-operative pain and required lower doses and duration of post-operative opioid analgesia. Furthermore, their functional recovery, as measured by time to first mobilization, positive response to straight leg raising and time to tolerance of 30–50° knee flexion, was superior.
The factors affecting the amount of post-operative pain experienced by patients after arthroscopic knee surgery and the quality of intra-articular analgesia include pre-operative pain levels, mode of anesthesia, surgical technique, duration of surgery and the volume and choice of intra-articular drugs [8]. We avoided potential bias by using spinal anesthesia for all participants, the same volume of intra-articular drugs and the same surgeon and surgical procedure, with the result that there was no significant difference in operation times between the groups.
Bupivacaine is the drug most frequently used for intraarticular analgesia because of its long duration of action [9]. Hansen et al. [9] found that there were clear differences in the post-operative pain scores between patients receiving intraarticular bupivacaine after arthroscopic shoulder surgery and those that received placebo. This finding was replicated by Morgenthaler et al. [10] who reported lower pain scores and also earlier mobilization times in patients receiving intra-articular bupivacaine after arthroscopic hip surgery compared with placebo. Woods and O’Connor [11] found that a single dose of intra-articular bupivacaine-morphine at the end of surgery provided analgesia as effectively as continuous femoral nerve block after anterior cruciate ligament reconstruction.
Bupivacaine is cardiotoxic, and as a result, the use of less toxic local anesthetics is becoming more widespread. Bupivacaine cardiotoxicity is not a major risk factor in arthroscopic knee surgery; however, minimizing risks, especially in outpatient surgery, is very important [12]. Sullivan et al. [13] reported two patients who experienced cardiac toxicity after 75–150 mg intra-articular bupivacaine, which is considered to be a safe dose. Liguori et al. [14] reported local anesthetic toxicity manifested as chest pain, nausea, faintness and tachycardia in a patient who received intra-articular bupivacaine (20 mL in 0.25% concentration) after arthroscopic knee surgery. Levobupivacaine is a new, long-acting amide local anesthetic and is the S (-) isomer of racemic bupivacaine. Clinical studies have shown that the anesthetic and analgesic effects of levobupivacaine are very similar to bupivacaine at the same dose [15–17]. In this study, we compared the efficacy of levobupivacaine, which is accepted to be safer in terms of side effects, with bupivacaine and found no differences in terms of their post-operative benefits. The hemodynamic profiles of the groups receiving local anesthetics were similar, and no complications were encountered.
The dose of intra-articular bupivacaine used in studies of post-operative pain control ranges from 20–40 ml (90±34 mg) of the 0.25% and 0.5% concentrations [18]. The dose clearly influences the quality of analgesia; 20 mL 0.25% bupivacaine provides approximately 2 hours of post-operative analgesia [19]. Joshi et al. [20] found that analgesia lasted approximately 4 hours with 25 mL intra-articular 0.25% bupivacaine. We used 30 mL 0.5% local anesthetic and achieved 258.5±19.8 and 251.50±17.4 minutes analgesia in the bupivacaine and levobupivacaine groups, respectively. Dose-response studies are required to determine the optimum volume and concentration for analgesia and to minimize side effects.
Few studies have examined the role of intra-articular levobupivacaine in providing post-operative analgesia, and the optimal dose has not yet been determined. Jacobson et al. [21] compared two groups that received intra-articular levobupivacaine at 0.25% and 0.5% concentrations with another group that received intra-articular 1% lidocaine with adrenaline (total volume 20 mL). They found that 0.5% levobupivacaine provided better analgesia in the first 24 post-operative hours than 0.25% levobupivacaine and the lidocaine-adrenaline combination.
Tetzlaff et al. [22] evaluated the effect of adding morphine, fentanyl or sufentanil to 20 mL 0.25% bupivacaine on postoperative analgesia after arthroscopic shoulder surgery and found that the morphine-bupivacaine combination was superior. Vasanth et al. [23] administered 2 mg intra-articular morphine in 20 mL normal saline to patients having arthroscopic knee surgery after general anesthesia and included two further groups who received 20 mL 0.25% bupivacaine and 20 mL 0.25% bupivacaine with 2 mg morphine, respectively; the combination of local anesthetic and morphine was found to be most effective. Stein et al. [24, 25] showed that 1 mg intraarticular morphine acts upon on peripheral opioid receptors and can be reversed with naloxone. However, the onset of analgesia after administration of intra-articular morphine is slow, providing weak pain relief in the first 2 hours and only having a meaningful therapeutic effect after 4 hours. Aasbo et al. [26] compared the post-operative analgesic effects of intra-articular bupivacaine and morphine and found that bupivacaine was effective within the first 2 post-operative hours, whereas the effect of morphine began after 2 hours, and its analgesic effect only reached peak levels in the sixth hour. By using 2 mg morphine, we aimed to achieve effective long-term post-operative analgesia with local anesthetic acting in the early post-operative period.
Adrenaline acts to prolong analgesia by causing vasoconstriction and reducing vascular absorption. Moreover, vasoconstriction also reduces the risk of intra- and post-operative hemorrhage. Gyrn et al. [27] showed that three combinations of bupivacaine and adrenaline (25 mg+50 µg, 50 mg+100 µg and 75 mg+150 µg, in a total volume of 30 mL) had no significant differences in terms of post-operative pain, analgesic requirement or surgeon satisfaction after knee arthroscopy. Similar findings have been reported after knee arthroplasty. Lombardi et al. [28] found that intra-operative intra-articular 0.25% bupivacaine with morphine and adrenaline provided better early post-operative analgesia and reduced blood loss and overall analgesic requirements. In our study, we chose to add morphine and adrenaline to reduce the overall dose of local anesthetic required.
In conclusion, levobupivacaine in combination with morphine and adrenaline was found to be as effective as bupivacaine with morphine and adrenaline when administered into the knee joint after ambulatory knee arthroscopic surgery under spinal anesthesia. Both combinations reduced postoperative pain, hastened the return of post-operative function and maintained vital parameters. We did not detect bupivacaine-related toxicity at this dose, but given the more benign toxicity profile of levobupivacaine, we recommend that the levo-isomer should be used in routine clinical practice.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Jackson RW. A history of arthroscopy. Arthroscopy. 2010;26:91–103. doi: 10.1016/j.arthro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Kieser CW, Jackson RW. Eugen Bircher (1882–1956) the first knee surgeon to use diagnostic arthroscopy. Arthroscopy. 2003;19:771–6. doi: 10.1016/s0749-8063(03)00693-5. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M. Memories of the early days of arthroscopy. Arthroscopy. 1986;2:209–14. doi: 10.1016/s0749-8063(86)80073-1. [DOI] [PubMed] [Google Scholar]
- 4.Coward DB. Principles of arthroscopy of the knee Chapman’s Orthopaedic Surgery. 3rd Edition. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 154–67. [Google Scholar]
- 5.Reuben SS, Ekman EF. The effect of initiating a preventive multimodal analgesic regimen on long-term patient outcomes for outpatient anterior cruciate ligament reconstruction surgery. Anesth Analg. 2007;105:228–32. doi: 10.1213/01.ane.0000265443.20919.c8. [DOI] [PubMed] [Google Scholar]
- 6.Ng HP, Nordström U, Axelsson K, et al. Efficacy of intra-articular bupivacaine, ropivacaine, or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: a randomized double-blind study. Reg Anesth Pain Med. 2006;31:26–33. doi: 10.1016/j.rapm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Gentili M, Houssel P, Osman M, Henel D, Juhel A, Bonnet F. Intraarticular morphine and clonidine produce comparable analgesia but the combination is not more effective. Br J Anaesth. 1997;79:660–1. doi: 10.1093/bja/79.5.660. [DOI] [PubMed] [Google Scholar]
- 8.Karaman Y, Kayalı C, Ozturk H, Kaya A, Bor C. A comparison of analgesic effect of intra-articular levobupivacaine with bupivacaine following knee arthroscopy. Saudi Med. 2009;30:629–32. [PubMed] [Google Scholar]
- 9.Hansen TB, Jakobsen IA. Intra-articular bupivacaine as treatment for postoperative pain after arthroscopy of the wrist. Scand J Plast Reconstr Surg Hand Surg. 2008;42:313–5. doi: 10.1080/02844310802271170. [DOI] [PubMed] [Google Scholar]
- 10.Morgenthaler K, Bauer C, Ziegeler S, et al. Intra-articular bupivacaine following hip joint arthroscopy. Effect on postoperative pain. Anaesthesist. 2007;56:1128–32. doi: 10.1007/s00101-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 11.Woods GW, O’Connor DP, Calder CT. Continuous femoral nerve block versus intra-articular injection for pain control after anterior cruciate ligament reconstruction. Am J Sports Med. 2006;34:1328–33. doi: 10.1177/0363546505286145. [DOI] [PubMed] [Google Scholar]
- 12.Raj N, Sehgal A, Hall JE, et al. Comparison of analgesic efficacy and plasma concentrations of high-dose intra-articular and intramuscular morphine for knee arthroscopy. Eur J Anaesthesiol. 2004;21:932–37. doi: 10.1017/s0265021504000304. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan SG, Abbott PJ., Jr Cardiovascular toxicity associated with intraarticular bupivacaine. Anesth Analg. 1994;79:591–3. doi: 10.1213/00000539-199409000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Liguori GA, Chimento GF, Borow L, Figgie M. Possible bupivacaine toxicity after intraarticular injection for postarthroscopic analgesia of the knee: implications of the surgical procedure. Anesth Analg. 2002;94:1010–3. doi: 10.1097/00000539-200204000-00044. [DOI] [PubMed] [Google Scholar]
- 15.Bardsley H, Gristwood R, Watson N, Nimmo W. The local anaesthetic activity of levobupivacaine does not differ from racemic bupivacaine (Marcain): first clinical evidence. Expert Opin Invest Drug. 1997;6:1883–5. doi: 10.1517/13543784.6.12.1883. [DOI] [PubMed] [Google Scholar]
- 16.Lyons G, Columb M, Wilson RC, Johnson RV. Epidural pain relief in labour: Potencies of levobupivacaine and rasemic bupivacaine. Br J Anaesth. 1998;81:899–901. doi: 10.1093/bja/81.6.899. [DOI] [PubMed] [Google Scholar]
- 17.Ivani G, Borghi B, van Oven H. Levobupivacaine. Minerva Anesthesiol. 2001;67:20–3. [PubMed] [Google Scholar]
- 18.Moiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Reg Anesth Pain Med. 1999;24:430–37. doi: 10.1016/s1098-7339(99)90010-x. [DOI] [PubMed] [Google Scholar]
- 19.Chirwa SS, MacLeod BA, Day B. Intraarticular bupivacaine (Marcaine) after arthroscopic meniscectomy: a randomized double-blind controlled study. Arthroscopy. 1989;5:33–5. doi: 10.1016/0749-8063(89)90087-x. [DOI] [PubMed] [Google Scholar]
- 20.Joshi GP, McCarroll SM, O’Brien TM, Lenane P. Intraarticular analgesia following knee arthroscopy. Anesth Analg. 1993;26:333–6. [PubMed] [Google Scholar]
- 21.Jacobson E, Assareh H, Cannerfelt R, Anderson RE, Jakobsson JG. The postoperative analgesic effects of intraarticular levobupivacaine in elective day-case arthroscopy of the knee: a prospective, randomized, double-blind clinical study. Knee Surg Sports Traumatol Arthrosc. 2006;14:120–24. doi: 10.1007/s00167-005-0655-4. [DOI] [PubMed] [Google Scholar]
- 22.Tetzlaff JE, Brems J, Dilger J. Intraarticular morphine and bupivacaine reduces postoperative pain after rotator cuff repair. Reg Anesth Pain Med. 2000;25:611–4. doi: 10.1053/rapm.2000.8573. [DOI] [PubMed] [Google Scholar]
- 23.Vasanth R, Sanjay OP, Latha J. Intra-articular administration of morphine, bupivacaine and morphine with bupivacaine for post-operative analgesia following video knee arthroscopy. Indian J Anaesth. 2003;47:265–8. [Google Scholar]
- 24.Stein C, Yassouridis LA. Peripheral morphine analgesia. Pain. 1997;71:119–21. [PubMed] [Google Scholar]
- 25.Stein C, Pflüger M, Yassouridis A, et al. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98:793–99. doi: 10.1172/JCI118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aasbø V, Raeder JC, Grøgaard B, Røise O. No additional analgesic effect of intraarticular morphine or bupivacaine compared with placebo after elective knee arthroscopy. Acta Anaesthesiol Scand. 1996;40:585–8. doi: 10.1111/j.1399-6576.1996.tb04492.x. [DOI] [PubMed] [Google Scholar]
- 27.Gyrn JP, Olsen KS, Appelquist E, et al. Intra-articular bupivacaine plus adrenaline for arthroscopic surgery of the knee. Acta Anaesthesiol Scand. 1992;36:643–6. doi: 10.1111/j.1399-6576.1992.tb03535.x. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;428:125–30. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]


