Abstract
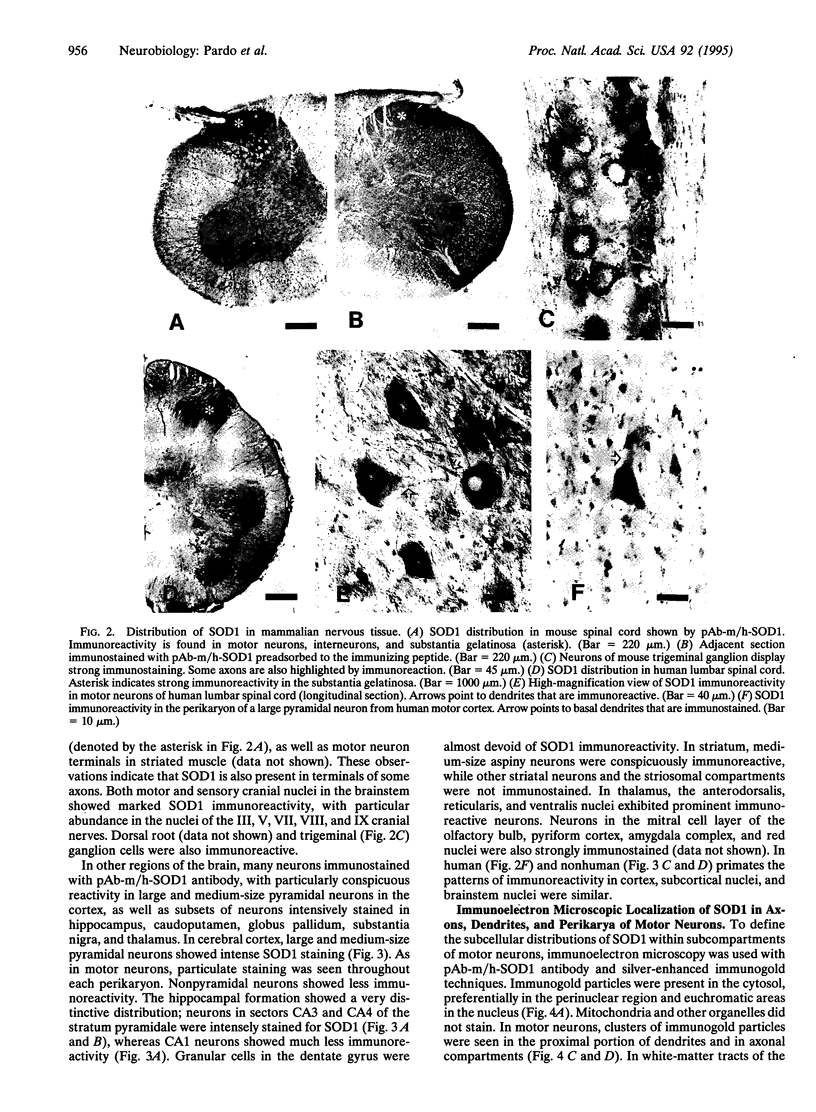
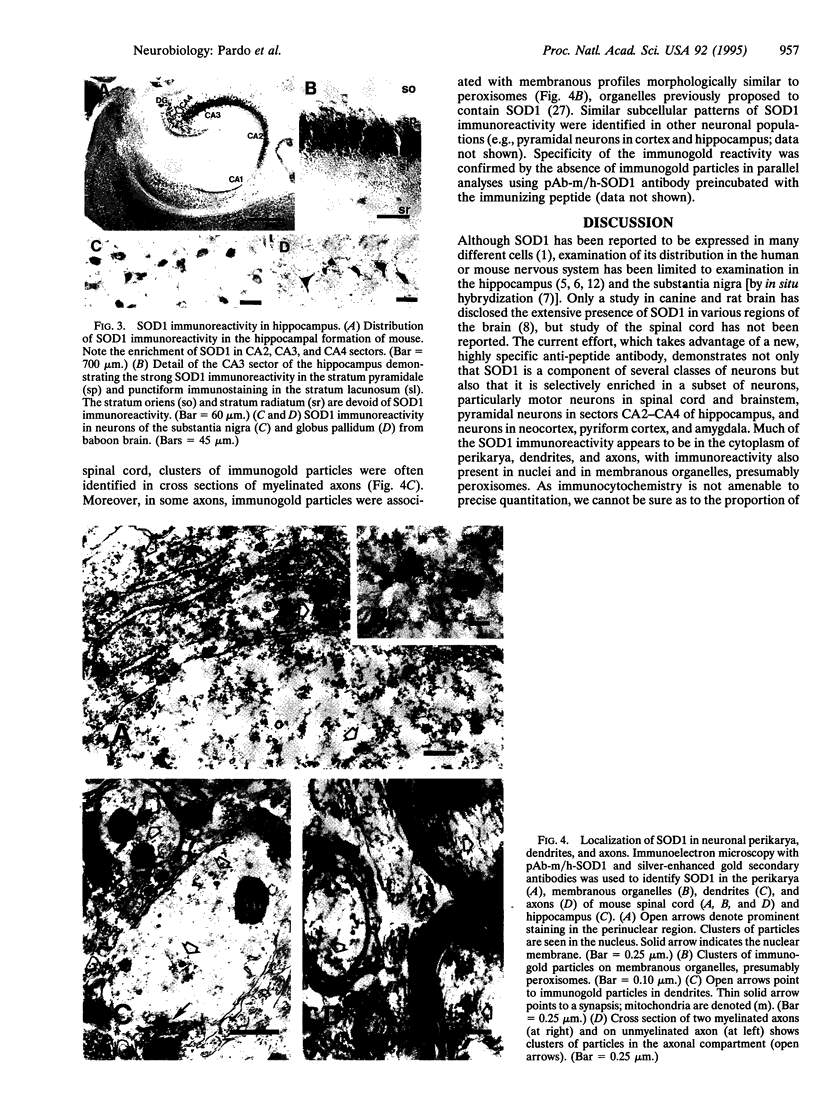
Mutation in superoxide dismutase 1 (SOD1), a Cu/Zn enzyme that removes oxygen radicals and protects against oxidative injury, has been implicated in some cases of familial amyotrophic lateral sclerosis (FALS). As a first approach to examining the mechanism(s) through which these mutations cause specific degeneration of motor neurons, we have used immunocytochemistry to identify the distribution of SOD1 in populations of cells in the peripheral and central nervous systems. In the spinal cord, intense SOD1 immunoreactivity was present in motor neurons, interneurons, and substantia gelatinosa. In motor neurons, SOD1 immunoreactivity was abundant in perikarya, dendrites, and axons; most of this activity appeared to be free in the cytoplasm, although a portion was associated with membranous vesicles, presumably peroxisomes. Since a variety of central nervous system neurons, including pyramidal cells in cerebral cortex and neurons of the CA3 and CA4 sectors of the hippocampus, showed high immunoreactivity but are unaffected in ALS, the apparent abundance of SOD1 does not predict vulnerability of neurons to mutations in SOD1. Rather, SOD1 accumulates in many neuronal populations but is particularly abundant in motor neurons. Consistent with recent studies of FALS-linked SOD1 mutations in vitro and in transgenic mice, our findings offer further support for the view that the mutations confer a gain of adverse function. In this view, high, rather than limiting, levels of SOD1 may place motor neurons selectively at risk in FALS.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bewley G. C. cDNA and deduced amino acid sequence of murine Cu-Zn superoxide dismutase. Nucleic Acids Res. 1988 Mar 25;16(6):2728–2728. doi: 10.1093/nar/16.6.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr, Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. C., Schulz J. B., Brown R. H., Jr, Beal M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993 Dec;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Ceballos I., Javoy-Agid F., Delacourte A., Defossez A., Lafon M., Hirsch E., Nicole A., Sinet P. M., Agid Y. Neuronal localization of copper-zinc superoxide dismutase protein and mRNA within the human hippocampus from control and Alzheimer's disease brains. Free Radic Res Commun. 1991;12-13 Pt 2:571–580. doi: 10.3109/10715769109145832. [DOI] [PubMed] [Google Scholar]
- Ceballos I., Javoy-Agid F., Hirsch E. C., Dumas S., Kamoun P. P., Sinet P. M., Agid Y. Localization of copper-zinc superoxide dismutase mRNA in human hippocampus by in situ hybridization. Neurosci Lett. 1989 Oct 23;105(1-2):41–46. doi: 10.1016/0304-3940(89)90008-6. [DOI] [PubMed] [Google Scholar]
- Chan J., Aoki C., Pickel V. M. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990 Aug;33(2-3):113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriolo M. R., Fiskin K., De Martino A., Corasaniti M. T., Nistico G., Rotilio G. Age-related changes in Cu,Zn superoxide dismutase, Se-dependent and -independent glutathione peroxidase and catalase activities in specific areas of rat brain. Mech Ageing Dev. 1991 Dec 31;61(3):287–297. doi: 10.1016/0047-6374(91)90061-4. [DOI] [PubMed] [Google Scholar]
- Collins R. C., Dobkin B. H., Choi D. W. Selective vulnerability of the brain: new insights into the pathophysiology of stroke. Ann Intern Med. 1989 Jun 15;110(12):992–1000. doi: 10.7326/0003-4819-110-12-992. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Defossez A., Ceballos I., Nicole A., Sinet P. M. Preferential localization of copper zinc superoxide dismutase in the vulnerable cortical neurons in Alzheimer's disease. Neurosci Lett. 1988 Oct 17;92(3):247–253. doi: 10.1016/0304-3940(88)90597-6. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Crapo J. D. cDNA and deduced amino acid sequence of rat copper-zinc-containing superoxide dismutase. Nucleic Acids Res. 1987 Aug 25;15(16):6746–6746. doi: 10.1093/nar/15.16.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y., Dyck P. J., Shimono M., Okazaki H., Tateishi J., Doi H. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1981 Nov;40(6):667–675. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Warner T. G., Steimer K. S., Hallewell R. A. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W. In vivo microtubules are copolymers of available beta-tubulin isotypes: localization of each of six vertebrate beta-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol. 1987 Oct;105(4):1707–1720. doi: 10.1083/jcb.105.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Michishita H., Nakamura H., Tsuchiyama M., Shimizu S., Watanabe K., Sugita M. Induction of copper-zinc superoxide dismutase in gerbil hippocampus after ischemia. J Cereb Blood Flow Metab. 1993 Jan;13(1):135–144. doi: 10.1038/jcbfm.1993.16. [DOI] [PubMed] [Google Scholar]
- Perrin R., Briançon S., Jeandel C., Artur Y., Minn A., Penin F., Siest G. Blood activity of Cu/Zn superoxide dismutase, glutathione peroxidase and catalase in Alzheimer's disease: a case-control study. Gerontology. 1990;36(5-6):306–313. doi: 10.1159/000213215. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W. A., Brierley J. B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982 May;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Robberecht W., Sapp P., Viaene M. K., Rosen D., McKenna-Yasek D., Haines J., Horvitz R., Theys P., Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994 Jan;62(1):384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Semsei I., Rao G., Richardson A. Expression of superoxide dismutase and catalase in rat brain as a function of age. Mech Ageing Dev. 1991 Apr 1;58(1):13–19. doi: 10.1016/0047-6374(91)90116-h. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville M. J., Percy M. E., Bergeron C., Yoong L. K., Grima E. A., McLachlan D. R. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991 Jan;9(1-2):1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Thaete L. G., Crouch R. K., Nakagawa F., Spicer S. S. The immunocytochemical demonstration of copper-zinc superoxide dismutase in the brain. J Neurocytol. 1986 Jun;15(3):337–343. doi: 10.1007/BF01611436. [DOI] [PubMed] [Google Scholar]
- Werns S. W., Lucchesi B. R. Free radicals and ischemic tissue injury. Trends Pharmacol Sci. 1990 Apr;11(4):161–166. doi: 10.1016/0165-6147(90)90068-J. [DOI] [PubMed] [Google Scholar]
- Yu B. P. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994 Jan;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zemlan F. P., Thienhaus O. J., Bosmann H. B. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res. 1989 Jan 2;476(1):160–162. doi: 10.1016/0006-8993(89)91550-3. [DOI] [PubMed] [Google Scholar]
- Zhang P., Damier P., Hirsch E. C., Agid Y., Ceballos-Picot I., Sinet P. M., Nicole A., Laurent M., Javoy-Agid F. Preferential expression of superoxide dismutase messenger RNA in melanized neurons in human mesencephalon. Neuroscience. 1993 Jul;55(1):167–175. doi: 10.1016/0306-4522(93)90463-p. [DOI] [PubMed] [Google Scholar]