Abstract
Objective:
The present study was designed to investigate the dose-dependent protective effect of L-carnitine (LC) on thyroid hormone-induced oxidative stress in rat liver tissue.
Materials and Methods:
Twenty-one male Sprague Dawley rats were divided into four groups: control, hyperthyroidism, hyperthyroidism plus L-carnitine 100, and hyperthyroidism plus L-carnitine 500. Hyperthyroidism was induced in rats by injecting 250 μg of L-thyroxine/kg body weight/day for twenty consecutive days. The activities of catalase (CAT), glutathione peroxidase (GPX) and myeloperoxidase (MPO) and the level of malondialdehyde (MDA) were measured in liver homogenates.
Results:
The liver CAT, GPX and MPO activities were significantly lower in the hyperthyroid rats than in the control group. Treating hyperthyroid rats with both low-dose (100 mg/kg) and high-dose (500 mg/kg) L-carnitine for 10 days resulted in a marked increase in the activities of the antioxidant enzymes in the liver tissue.
Conclusion:
The present study indicates that the low-dose L-carnitine application was sufficient to prevent L-thyroxine-induced oxidative stress in rat livers.
Keywords: Catalase, glutathione peroxidase, L-carnitine, Liver, Malondialdehyde, Myeloperoxidase
Özet
Amaç:
Bu çalışma rat karaciğer dokusunda tiroid hormonu ile uyarılan oksidatif stres üzerine L-karnitinin doza bağlı koruyucu etkilerini araştırmaktı.
Gereç ve Yöntem:
Yirmi bir erkek Sprague Dawley rat 4 gruba bölündü: Kontrol, hipertroidi, hipertiroidi + L-karnitine 100 ve hipertiroidi + L-karnitine 500. Hipertiroidi, yirmi gün boyunca ratlara 250 μg / kg dozunda L-Thyroxine enjeksiyonu uygulanarak oluşturuldu. Karaciğer homojenatlarında katalaz (CAT), glutatyon peroksidaz (GPX), myeloperoksidaz (MPO) aktiviteleri ve malondialdehit (MDA) düzeyleri ölçüldü.
Bulgular:
Kontrollerle karşılaştırıldığında hipertiroidili ratlarda karaciğer CAT, GPX ve MPO aktiviteleri belirgin olarak azaldı. 10 gün boyunca hem düşük doz (100 mg/kg) hem de yüksek doz (500 mg/kg) L-karnitin uygulaması hipertiroidili ratlarda karaciğer antioksidan enzim aktivitelerinde belirgin bir artışa yol açtı.
Sonuç:
Bu çalışmanın sonuçları L-Tiroksin ile uyarılan rat karaciğerindeki oksidatif stresi önlemek için düşük doz L-karnitin uygulamasının yeterli olduğunu göstermektedir.
Introduction
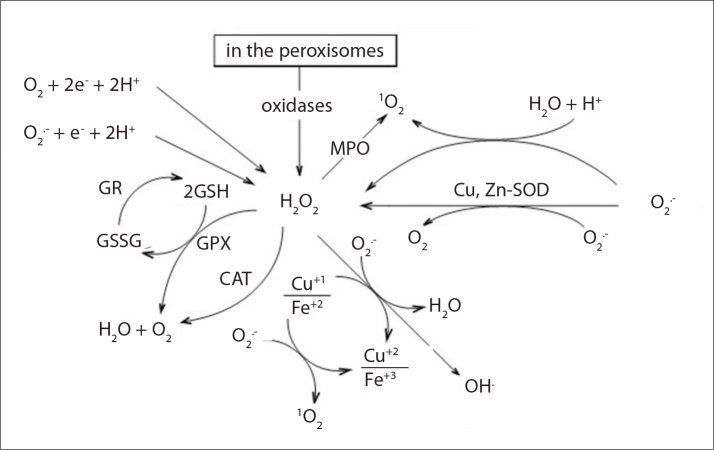
Thyroid hormones are the most important factor involved in setting the basal metabolic rate in target tissues such as the liver, heart, kidney and brain. Therefore, thyroid hormone administration to vertebrates leads to an accelerated basal metabolic rate and oxygen consumption in the target tissues [1]. Both clinical [2, 3] and experimental [1, 4, 5] studies have demonstrated that hyperthyroidism can cause an elevation in oxidative stress, most likely because of increased mitochondrial oxygen consumption. Mitochondria are the main production site of free oxygen radicals in healthy tissues because the leak of these radicals from the mitochondrial respiratory chain and the free oxygen radical production rate can be directly related to the rate of mitochondrial oxygen consumption [6]. The superoxide (O2−), hydroxyl (HO), perhydroxyl radicals (HO2−) and nitric oxide (NO) are the most important free radicals that are derived from oxygen [7]. If not immediately neutralized, O2− radicals are converted to other reactive oxygen species (ROS), such as the HO radical and H2O2, by a classical Fenton reaction in the presence of iron. Because of their high reactivity and non-specific nature, ROS can attack almost all biomolecules present in their vicinity, including membrane lipids, causing oxidative stress in cells [4]. However, the extracellular environment and cells have various antioxidant systems, including enzymatic and nonenzymatic antioxidant (e.g., L-carnitine) molecules. Glutathione peroxidase (GPX), catalase (CAT) and myeloperoxidase (MPO) play an important role in the natural enzymatic defense system that detoxifies H2O2 in water (Figure 1) [8, 9].
Figure 1.
Antioxidant enzymes that detoxify hydrogen peroxide (H2O2). GPX, CAT and MPO play an important role in the natural enzymatic defense system for detoxifying H2O2 in water. GPX, glutathione peroxidase; GR, glutathione reductase; CAT, catalase; MPO, myeloperoxidase; GSSG, oxidized glutathione; GSH, reduced glutathione; O2·−, superoxide; OH·, hydroxyl radical; Cu,Zn-SOD, Cu,Zn- superoxide dismutase; 1O2, singlet oxygen.
L-carnitine (4-N-trimethylammonium-3-hydroxybutyric acid) is a natural nutrient that transports long-chain fatty acids into the mitochondria, where they are oxidized to produce adenosine triphosphate [10] and prevent the toxic accumulation of long-chain fatty acids [11]. L-carnitine inhibits both the mitochondrial damage induced by oxidative stress and mitochondria-dependent apoptosis in various types of cells [12, 13]. Recent studies suggest that L-carnitine may play an important role in oxidative/antioxidative balance and has an antiperoxidative effect on several tissues [14–16].
Recent studies have provided considerable support for the protective effects of L-carnitine against oxidative stress. However, to our knowledge, there is a lack of information in the literature on the dose-dependent protective effect of L-carnitine against the free radical damage induced by hyperthyroidism in rat liver tissue. In this study, we aimed to investigate the dose-dependent protective effect of L-carnitine on thyroid hormone-induced oxidative stress. For this purpose, we designed an experimental study and measured the enzyme activities of GPX, CAT and MPO and the level of malondialdehyde (MDA) in liver homogenates from rats.
Materials and Methods
Adult male Sprague Dawley rats (258±23 g, 8–10 weeks old) were obtained from the Experimental Research Centre of the Atatürk University, Erzurum, Turkey. The animals were housed in metal cages under standard laboratory conditions (controlled temperature of 22–24°C, 12h light/dark cycle) and received water and pelleted food ad libitum. This experimental study was conducted with the approval of the Local Ethics Board of Animal Experiments in Ataturk University.
Experimental procedure
Age-matched rats were divided into four groups and treated as follows. The first group of rats (n=6) served as the control (C). The control rats were injected with corresponding volumes (0.2 ml/100g body weight, subcutaneously) of 0.9% NaCl solution. In the second group (Hyper, n=5), hyperthyroidism was induced with L-thyroxine (Sigma T1775), 250 mg/kg body weight administered subcutaneously for twenty consecutive days [17]. The L-thyroxine solution was prepared as described previously [18]. In the third group (Hyper+LC100, n=5), hyperthyroidism was induced as in the second group. In addition, to test the effect of a lower dose of L-carnitine, L-carnitine treatment was started with a dose of 100 mg/kg/day body weight intraperitoneally (i.p.) to the rats on the tenth day of hyperthyroidism and was continued for the next ten days. In the fourth group (Hyper+LC500, n=5), hyperthyroidism was generated as in the second group. However, to test the effect of a higher dose of L-carnitine, the L-carnitine treatment was started at a dose of 500 mg/kg/day body weight i.p. on the tenth day of hyperthyroidism and continued for the next ten days. All injections were performed between 10 and 11 AM. During the course of the treatment, the body weights of all rats were periodically measured at the same time of day.
At the end of the experimental procedures, all animals were sacrificed by drawing blood directly from the heart under ether anesthesia on the twenty-first day after the initiation of the study. Liver tissues were rapidly excised, washed with cold 0.9% NaCl solution, blotted, and then frozen at −80°C.
Tissue preparation and biochemical assays
For biochemical assays, the liver tissues were homogenized (10%, w/v) in ice-cold buffers that were appropriate for the variable to be measured, as described previously [7, 19]. The homogenization procedures were performed at 4°C in an OMNI-TH homogenizer (Warrenton, VA, USA). The tissue homogenates were centrifuged at 15,000 g for 15 min at 4°C, and the supernatants were extracted to analyze GPX [7], CAT [7], and MPO [19]. The oxidative stress status in the liver homogenates was determined by measuring the level of MDA according to the method described by Yildirim et al. [7] The protein concentrations in the homogenate and supernatant were determined using the Bradford method [20]. The results for GPX and MPO, CAT, and MDA were expressed as U/g protein, k/g protein, and µM/g protein, respectively.
Statistical analysis
For statistical analysis, differences between the groups were tested using the Wilcoxon and the Mann-Whitney U tests in SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered significant. All data were expressed as the mean±standard deviation (SD).
Results
The changes in the body weights of the rats during the study periods were summarized in Table 1. The average gain in weight of the rats in the control group during the 21-day period was 49 grams. As expected, a loss of weight averaging 24 grams was observed in the rats treated with L-thyroxine. In contrast, the rats treated with L-carnitine exhibited a slight increase in body weight throughout the 21-day experimental periods (Table 1).
Table 1.
Evolution of the body weights, assessed on day 1 and on day 21 (sacrifice day), in rats and differences (Δ, %) relative to the initial body weight (values are the mean±SD)
| Groups | Body Weights (g) | ||
|---|---|---|---|
| Initial (Day 1) | Sacrifice day (Day 21) | Differences (Δ, %) | |
| Control (n=6) | 257±22 | 306±20a | +49 g, 19.1% |
| Hyper (n=5) | 278±30 | 254±18b | −24 g, 8.6% |
| Hyper+LC100 (n=5) | 255±12 | 261±11 | +6 g, 2.4% |
| Hyper+LC500 (n=5) | 245±19 | 255±17 | +10 g, 3.9% |
p<0.001 and
p< 0.05 vs. Day 1 (Wilcoxon Test results)
As shown in Figures 2, 3, and 4, the liver CAT, GPX and MPO activities decreased to a significant extent in the hyper-thyroid rats when compared with the control group (76, 40, and 21%, respectively). In contrast, when compared with the controls, the hyperthyroidism in the second group of rats caused a marked increase in the levels of hepatic MDA (59%, P<0.001). However, this increase in MDA levels was prevented by the treatment with L-carnitine (Figure 5).
Figure 2.
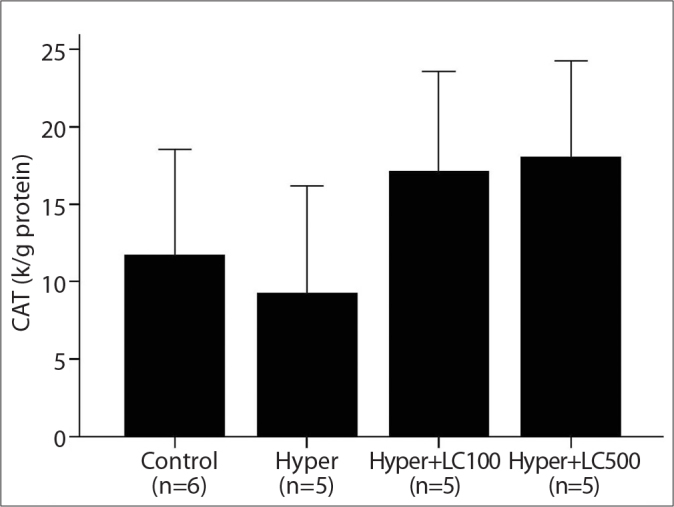
The liver CAT activities in the studied groups. Hyper, hyperthyroidism; Hyper+LC100, hyperthyroidism+L-carnitine 100 mg/kg; Hyper+LC500, hyperthyroidism+L-carnitine 500 mg/kg. *p<0.05 vs. Hyper (Mann-Whitney Test results).
Figure 3.
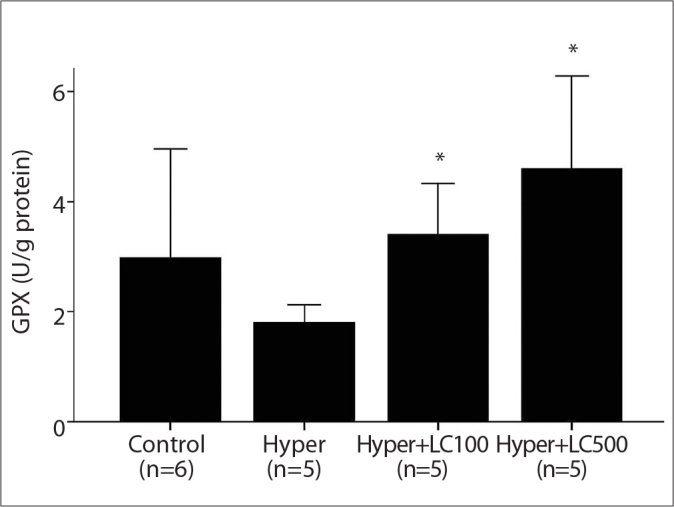
The liver GPX activities in the studied groups. Hyper, hyperthyroidism; Hyper+LC100, hyperthyroidism+L-carnitine 100 mg/kg; Hyper+LC500, hyperthyroidism+L-carnitine 500 mg/kg. *p<0.005 vs. Hyper (Mann-Whitney Test results).
Figure 4.
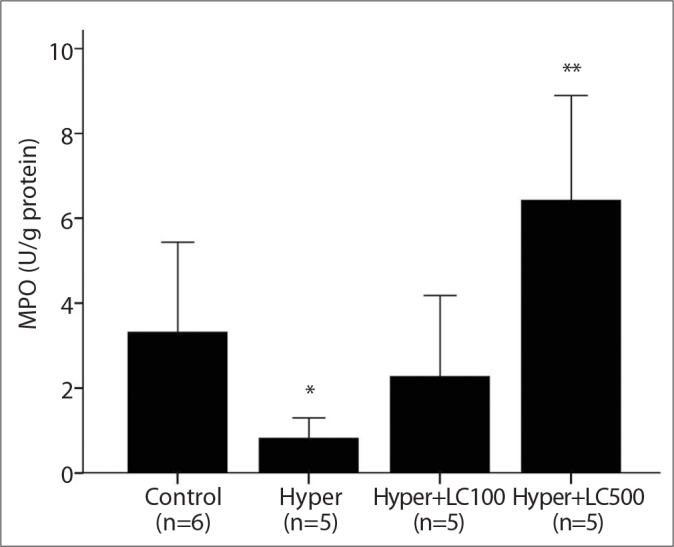
The liver MPO activities in the studied groups. Hyper, hyperthyroidism; Hyper+LC100, hyperthyroidism+L-carnitine 100 mg/kg; Hyper+LC500, hyperthyroidism+L-carnitine 500 mg/kg. *p<0.01 vs. Control, **p<0.001 vs. Hyper (Mann-Whitney Test results).
Figure 5.
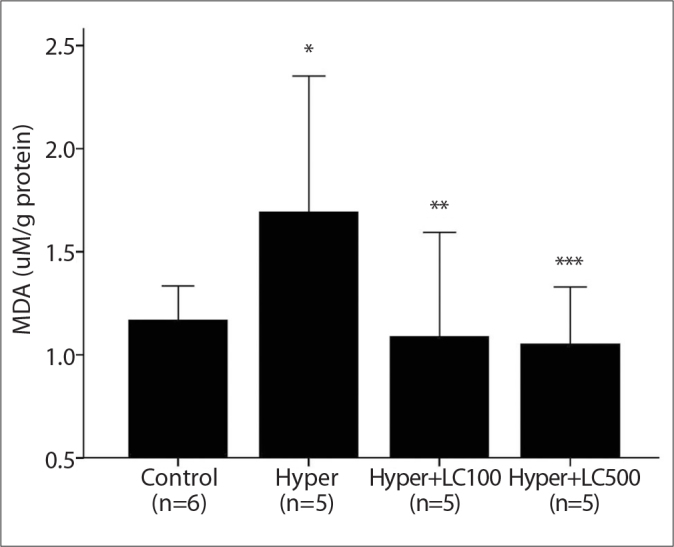
The liver MDA levels in the studied groups. Hyper, hyperthyroidism; Hyper+LC100, hyperthyroidism+L-carnitine 100 mg/kg; Hyper+LC500, hyperthyroidism+L-carnitine 500 mg/kg. *p<0.001 vs. Control, **p<0.05 and ***p<0.005 vs. Hyper (Mann-Whitney Test results).
Furthermore, treating the hyperthyroid rats with both low-dose (100 mg/kg) and high-dose (500 mg/kg) L-carnitine for 10 days resulted in a marked increase in the antioxidant enzyme activities in the liver tissue when compared with levels in the rats in the second group. These L-carnitine-induced increases in the activities of MPO, GPX and CAT were more prominent in the high-dose L-carnitine group (Hyper+LC500) than in the low-dose group (Hyper+LC100), but the differences between the groups was not statistically significant (Figures 2, 3, and 4).
Discussion
Oxidative stress occurs when cellular antioxidant defense systems are insufficient to keep the levels of ROS below a toxic threshold, which may result from excessive production of ROS, the failure of antioxidant defenses, or both [21]. Earlier experimental and clinical studies have shown that hyperthyroidism caused both an elevation in the production of free oxygen radicals and an abnormal oxidative status of the organism [4, 22]. Rybus-Kalinowska et al. [3] reported that the pre-treatment levels of plasma MDA, which was used as an oxidative stress biomarker, were significantly higher in women with hyperthyroidism and that antithyroid therapy led to the normalization of the plasma MDA levels. Additionally, in another study, Messarah et al. [5] demonstrated that the liver MDA contents rats with experimental hyperthyroidism significantly increased compared with those in the controls. Our results showed that L-thyroxine administration caused a marked increase in the levels of hepatic MDA, as stated in the introduction, most likely by accelerating the basal metabolic rate and oxygen consumption in the target tissues [1].
L-Carnitine is produced from both dietary sources (75%) and endogenous biosynthesis (25%) in the human body [23]. Endogenous L-carnitine is synthesized from lysine and methionine primarily in the liver, as well as in the kidneys and brain tissue [24]. Previous studies have indicated that L-carnitine has an important role in oxidative/antioxidative balance as well as in the transport function of long-chain fatty acids into the mitochondria in biological systems [12–15]. However, the effects of L-carnitine on specific antioxidant enzymes, such as GPX, CAT and MPO, which detoxify H2O2 in water, have been reported independently in a few studies [14, 25]. There are no studies about the dose-dependent protective effect of L-carnitine against the free radical damage induced by hyperthyroidism in rat liver tissue.
Cayır et al. [14] examined the effects of L-carnitine at a dose of 500 mg/kg (i.p.) on cisplatin-induced oxidative damage in the liver and kidney tissues of rats. Their data showed that L-carnitine elicited significant protective activity in the liver and kidney by decreasing the levels of MDA and elevating the activities of GPX, suggesting an antioxidant effect from this molecule. This finding was most likely caused by a decrease in the damage produced by free oxygen radicals [14]. Cetinkaya et al. [25] investigated the effects of L-carnitine at a dose of 500 mg/kg (i.p.) on the oxidant/antioxidant status in acetic acid-induced colitis and reported that L-carnitine administration to the acetic acid-treated rats significantly reduced the MDA level and MPO activity, whereas the CAT activity increased in colon tissue. In contrast to the results of Cetinkaya, in our study, two different doses of administered L-carnitine significantly induced the MPO activities in liver tissue. The different results from our data might result from the acetic acid-induced inflammatory process. MPO is a heme-containing peroxidase abundantly expressed in neutrophils. In addition, enzymatically active MPO, together with hydrogen peroxide and chloride, produces the powerful oxidant hypochlorous acid and is a key contributor to the oxygen-dependent microbicidal activity of phagocytes. Therefore, the change in MPO activity can vary depending on whether the number of neutrophil leukocytes increases, as in the case of inflammation. However, in our study, an inflammatory process was not stimulated experimentally.
Aleisa et al. [13] reported that the administration of propionyl L-carnitine at a dose of 250 mg/kg (i.p.) significantly attenuated the nephrotoxic effects of cisplatin, which manifested as a normalization of the cisplatin-induced increase in thiobarbituric acid reactive substances, such as MDA and nitric oxide, and the cisplatin-induced decrease in reduced glutathione in rat kidney tissues. In another study, Derin et al. [26] reported that pretreatment with L-carnitine (100 mg/kg, i.p.) increased the tissue catalase activity and protected the gastric mucosa from ischemia-reperfusion injury by decreasing lipid peroxidation via its lipid peroxidation-decreasing activity. As in earlier reports, in the present study, L-carnitine supplementation at doses of 100 and 500 mg/kg caused a significant decrease in the hepatic lipid peroxidation and an increase in the activities of GPX, CAT and MPO in rat liver tissue. However, there was no significant difference in the reduction of the L-thyroxine-induced oxidative stress between the groups supplemented with low- and high-dose carnitine.
In conclusion, L-thyroxine administration (250 mg/kg, s.c.) caused a marked increase in the MDA levels in the rat liver. A low-dose L-carnitine (100 mg/kg body weight, i.p.) application was sufficient to prevent the L-thyroxine-induced oxidative stress. These effects of L-carnitine may result directly from antioxidant effects against free oxygen radicals or from enhanced biosynthesis of enzymatic antioxidants, such as GPX, CAT and MPO.
Acknowledgments
This work was financially supported by the Ataturk University Research Council (Grant number: 2010/114).
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Venditti P, Bari A, Di Stefano L, Di Meo S. Effect of T3 on metabolic response and oxidative stress in skeletal muscle from sedentary and trained rats. Free Radic Biol Med. 2009;46:360–6. doi: 10.1016/j.freeradbiomed.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Akarsu E, Buyukhatipoglu H, Aktaran S, Kurtul N. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2011;74:118–24. doi: 10.1111/j.1365-2265.2010.03904.x. [DOI] [PubMed] [Google Scholar]
- 3.Rybus-Kalinowska B, Zwirska-Korczala K, Kalinowski M, Kukla M, Birkner E, Jochem J. Activity of antioxidative enzymes and concentration of malondialdehyde as oxidative status markers in women with newly diagnosed Graves-Basedow disease and after thiamazole therapy leading to euthyroidism. Pol Arch Med Wewn. 2008;118:420–5. [PubMed] [Google Scholar]
- 4.Das K, Chainy GB. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim Biophys Acta. 2001;1537:1–13. doi: 10.1016/s0925-4439(01)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Messarah M, Boumendjel A, Chouabia A, et al. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp Toxicol Pathol. 2010;62:301–10. doi: 10.1016/j.etp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero A, Pamplona R, Portero-Otín M, Barja G, López-Torres M. Effect of thyroid status on lipid composition and peroxidation in the mouse liver. Free Radic Biol Med. 1999;26:73–80. doi: 10.1016/s0891-5849(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim A, Gumus M, Dalga S, Sahin YN, Akcay F. Dehydroepiandrosterone improves hepatic antioxidant systems after renal ischemia-reperfusion injury in rabbits. Ann Clin Lab Sci. 2003;33:459–64. [PubMed] [Google Scholar]
- 8.Yildirim A, Sahin YN, Suleyman H, Yilmaz A, Yildirim S. The role of prednisolone and epinephrine on gastric tissue and erythrocyte antioxidant status in adrenalectomized rats. J Physiol Pharmacol. 2007;58:105–16. [PubMed] [Google Scholar]
- 9.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–54. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinclair C, Gilchrist JM, Hennessey JV, Kandula M. Muscle carnitine in hypo- and hyperthyroidism. Muscle Nerve. 2005;32:357–9. doi: 10.1002/mus.20336. [DOI] [PubMed] [Google Scholar]
- 11.Ulvi H, Aygül R, Demir R. Effect of L-carnitine on diabetic neuropathy and ventricular dispersion in patients with diabetes mellitus. Turk J Med Sci. 2010;40:169–75. [Google Scholar]
- 12.Al-Majed AA. Carnitine deficiency provokes cisplatin-induced hepatotoxicity in rats. Basic Clin Pharmacol Toxicol. 2007;100:145–50. doi: 10.1111/j.1742-7843.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 13.Aleisa AM, Al-Majed AA, Al-Yahya AA, et al. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34:1252–9. doi: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- 14.Cayir K, Karadeniz A, Yildirim A, et al. Protective effect of L-carnitine against cisplatin-induced liver and kidney oxidant injury in rats. Cent Eur J Med. 2009;4:184–91. [Google Scholar]
- 15.Bayraktar N, Paşaoğlu H, Paşaoğlu A, Kaymaz M, Biberoğlu G, Kulaksizoğlu S. The relationship between carnitine levels and lipid peroxidation in glial brain tumors. Turk J Med Sci. 2008;38:293–9. [Google Scholar]
- 16.Pehlivan M, Coşkun A, Zengin A, Aslaner A, Yavuz T. Does L-carnitine increase serum TNF -α and IGF-1 during liver regeneration in the rat? Turk J Med Sci. 2009;39:875–80. [Google Scholar]
- 17.Ayala C, Valdez SR, Morero ML, et al. Hypo- and hyperthyroidism affect NEI concentration in discrete brain areas of adult male rats. Peptides. 2011;32:1249–54. doi: 10.1016/j.peptides.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Halapas A, Lembessis P, Mourouzis I, et al. Experimental hyperthyroidism increases expression of parathyroid hormone-related peptide and type-1 parathyroid hormone receptor in rat ventricular myocardium of the Langendorff ischaemia-reperfusion model. Exp Physiol. 2008;93:237–46. doi: 10.1113/expphysiol.2007.039594. [DOI] [PubMed] [Google Scholar]
- 19.Toth B, Schwacha MG, Kuebler JF, Bland KI, Wang P, Chaudrya IH. Gender dimorphism in neutrophil priming and activation following trauma-hemorrhagic shock. Int J Mol Med. 2003;11:357–64. [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Yildirim A, Kotan D, Yildirim S, Aygul R, Akcay F. Increased lipid peroxidation and decreased antioxidant response in serum and cerebrospinal fluid in acute ischemic stroke. Turk J Med Sci. 2007;37:75–81. [Google Scholar]
- 22.Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci. 2006;63:414–34. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siktar E, Ekinci D, Beydemir S, Gulcin I, Gunay M. Protective role of L-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. Eur J Pharmacol. 2011;668:407–13. doi: 10.1016/j.ejphar.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Rebouche CJ, Engel AG. Tissue distribution of carnitine biosynthetic enzymes in man. Biochim Biophys Acta. 1980;630:22–9. doi: 10.1016/0304-4165(80)90133-6. [DOI] [PubMed] [Google Scholar]
- 25.Cetinkaya A, Bulbuloglu E, Kantarceken B, et al. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–94. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- 26.Derin N, Izgut-Uysal VN, Agac A, Aliciguzel Y, Demir N. L-carnitine protects gastric mucosa by decreasing ischemia-reperfusion induced lipid peroxidation. J Physiol Pharmacol. 2004;55:595–606. [PubMed] [Google Scholar]