Abstract
Department of Life Science, The University of Seoul, Seoul 130-743, Korea Balanced cell growth is crucial in animal development as well as tissue homeostasis. Concerted cross-regulation of multiple signaling pathways is essential for those purposes, and the dysregulation of signaling may lead to a variety of human diseases such as cancer. The time-honored Wnt/β-catenin and recently identified Hippo signaling pathways are evolutionarily conserved in both Drosophila and mammals, and are generally considered as having positive and negative roles in cell proliferation, respectively. While most mainstream regulators of the Wnt/β-catenin signaling pathway have been fairly well identified, the regulators of the Hippo pathway need to be more defined. The Hippo pathway controls organ size primarily by regulating cell contact inhibition. Recently, several crossregulations occurring between the Wnt/β-catenin and Hippo signaling pathways were determined through biochemical and genetic approaches. In the present mini-review, we mainly discuss the signal transduction mechanism of the Hippo signaling pathway, along with cross-talk between the regulators of the Wnt/β-catenin and Hippo signaling pathways. [BMB Reports 2014; 47(10): 540-545]
Keywords: β-catenin, Crosstalk, Hippo signaling, Wnt signaling, YAP/TAZ
INTRODUCTION
Understanding the mechanisms for controlling the size of animals and their organs has been a challenging issue in biology, and the molecular mechanisms remain poorly understood (1-3). It is obvious that cell growth, proliferation, differentiation, and death should be tightly controlled to attain organs of the proper size during development, and that tissue homeostasis should be maintained in adults. Relatively recent studies suggested that the Hippo signaling pathway is a key mechanism for the control of organ size (1-5). The upstream regulators and the list of genes regulated by the Hippo pathway suggest that it negatively regulates cell proliferation (5). Uncontrolled cell proliferation due to dysregulation of Hippo signaling is responsible for tumor formation (1, 4, 6). Therefore, Hippo signaling is under intense scrutiny because of its significant roles in both developmental and cancer biology.
Cell growth and proliferation are also controlled by other well-known signaling pathways, such as Wnt/β-catenin and TGFβ signaling (7-9). Recent studies have proven that multiple signaling pathways cross-regulate each other to attain fine regulation of certain biological phenomenon. Specifically, it has recently been suggested that diverse signaling pathways such as Wnt/β-catenin (10-12), Shh (13), BMP/TGFβ (14-16), and GPCR signaling (17) cooperate with the Hippo signaling pathway to control cell growth and proliferation.
In this mini-review, we mainly describe recent advances in the Hippo signaling pathway, along with a brief explanation of the Wnt/β-catenin signaling pathway. Several examples of the merging of the two signaling pathways by unexpected cross-talk between components of the Wnt/β-catenin and Hippo signaling pathways, which may provide novel therapeutic targets for cancer treatment, are also discussed.
Wnt/β-CATENIN SIGNALING PATHWAY
Wnt signaling plays critical roles during embryonic development as well as in homeostatic mechanisms in adult tissues (7, 8). Complexity inferred by the temporal and spatial expression of 19 different Wnts and 10 types of Frizzled receptors, in mice and human, enables the Wnt signaling pathway to be involved in the control of diverse biological processes such as cell proliferation, differentiation, fate determination, adipogenesis, aging, etc (18, 19). Therefore, dysregulation of Wnt signaling can lead to diverse human diseases including cancers, osteoporosis, and neurodegeneration.
Wnt signaling can be divided into canonical (β-catenin dependent) and non-canonical (β-catenin independent) pathways based on whether increase of and nuclear localization of β-catenin occur in the presence of Wnt ligands (19, 20). Combinations of certain types of Wnt and Wnt receptors leads to the stabilization of β-catenin, while other combinations transduce signals via small G-proteins such as Rho/Rac or through regulation of the intracellular calcium level. Since most of the known cross-talk occurring between Hippo and Wnt signaling, the main theme of this review, are restricted to Wnt/β-catenin signaling, the canonical Wnt signaling pathway will be described in the present review. Outstanding reviews on non-canonical Wnt signaling are available elsewhere (21-23).
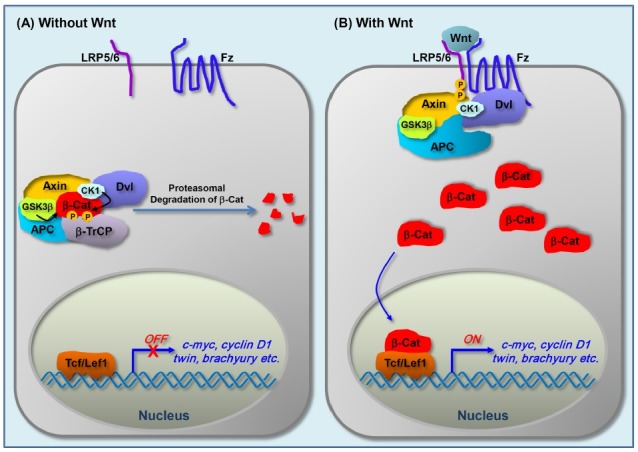
Wnts are highly conserved secreted proteins with glycosylation and lipid-modification, and act as ligands (24, 25). β-catenin is a transcriptional co-activator, and regulation of the level of and nuclear localization of β-catenin is a pivotal regulatory step in the Wnt/β-catenin signaling pathway. In the absence of Wnt, cytoplasmic β-catenin is consistently phosphorylated by GSK3β (glycogen synthase kinase 3β) in a destruction complex containing Axin and APC (adenomatous polyposis coli) (Fig. 1). The phosphorylated β-catenin is then ubiquitinated by the E3 ligase β-TrCP (β-transducin repeat-containing protein), and subsequently degraded in a proteasome-dependent manner to result in low cytoplasmic levels of β-catenin. The expression of genes regulated by Wnt/β-catenin signaling is thereby repressed due to the low levels of the transcriptional co-activator, β-catenin (Fig. 1A). However, in the presence of Wnt, binding of Wnt to its receptor Fz (Frizzled) and co-receptor LRP5/6 leads to phosphorylation of the intracellular region of LRP5/6 by GSK3β and CK1γ. Axin interacts with the phosphorylated LRP5/6 resulting in elevation in the levels of cytoplasmic β-catenin in a Dvl (Dishevelled)-dependent manner, though it is still controversial whether Axin translocates to the phosphorylated LRP5/6 apart from the components of the β-catenin destruction complex or as a whole complex (26, 27). The accumulated cytoplasmic β-catenin then enters into the nucleus and interacts with TCF (T-cell factor)/LEF (lymphoid enhancer factor) to activate the expression of Wnt target genes, which control cell proliferation (for example, c-myc and cyclin D1) and developmental processes (for example, twin, brachyuryetc.). Due to space limitations the basic frame of signal transduction of the Wnt/β-catenin pathway was explained only briefly; however, much more elaborated regulatory mechanisms can be found in recent reviews, including ours (7, 8, 19).
Fig. 1. Wnt/β-catenin signaling pathway. Schematic diagram for the core components and signal transduction of Wnt/β-catenin pathway. (A) In the absence of Wnt, GSK3β and CK1 phosphorylate β-catenin degradation complex which includes APC and Axin. The phosphorylated β-catenin is recognized by β-TrCP and subsequently degraded by proteasomal pathway. As a result, TCF/LEF1 suppresses the expression of target genes. (B) In the presence of Wnt, binding of Wnt to Fz and its co-receptor LRP5/6 leads to phosphorylation of LRP6. Axin, itself alone or whole β-catenin degradation complex including Axin, translocates to the phosphorylated LRP5/6, which leads to stabilization of cytoplasmic β-catenin. The stabilized β-catenin translocates into the nucleus and interacts with TCF/LEF1, which in turn enhances the expression of target genes.
HIPPO SIGNALING PATHWAY
Recent studies have shown that the Hippo signaling pathway is a conserved regulator of organ size. The Hippo signaling pathway is composed of a core kinase cascade initiating from Hippo (Mst1 and Mst2 in mammals) to the phosphorylation of a Yki (YAP and TAZ in mammals), which leads to change of the subcellular localization of Yki from the nucleus, where it acts as a transcriptional activator, to the cytoplasm (4, 28, 29). The Hippo signaling pathway does not have specifically allocated extracellular ligands or receptors, but instead appears to be regulated by a network of upstream components which are mainly involved in the regulation of cell adhesion and cell polarity (1, 30-33). It is evident that the core kinase cascade is strictly conserved, while the upstream signals influencing the kinase activity are much more diverse, for which the biochemical mechanisms of regulation are still obscure. The Hippo signaling pathway was also found to cross-talk with multiple signaling pathways in a tissue or context-dependent manner; therefore, it may be reasonable to consider Hippo signaling as a complex network rather than a single linear pathway.
The core kinase cascade for the inactivation of YAP/TAZ
Hippo signaling is composed of a highly conserved kinase cascade module that relays signals in a similar fashion in both mammals and Drosophila (1, 4). Mutations of the genes composing the core kinase complex, which were discovered in genetic screens in Drosophila for tumor suppressor genes, lead to a phenotype similar to hippopotamus in Drosophila due to increased cell proliferation and diminished cell death. As such, this signaling was named the Hippo pathway. The identified genes encode the following serine/threonine protein kinases and scaffolding proteins: (1) Hippo (MST1 and MST2 in mammals), which interacts with Salvador (SAV1 or also known as WW45 in mammals); and (2) Warts (LATS1 and LATS2 in mammals), which interacts with Mats (MOB1A and MOB1B in mammals) (1, 5). The transcriptional co-activator Yorkie (YAP and TAZ in mammals) forms a complex with the transcription factor Scalloped (TEAD 1-4 in mammals) to finally control the expression of genes regulated by the Hippo signaling pathway (Fig. 2).
Fig. 2. Hippo signaling pathway. Schematic diagram for the core components and signal transduction of Hippo pathway. (A) When Hippo signaling is Off (in low cell density): The kinases MST1/2 and LATS are inactive, which results in inhibition of phosphorylation of YAP and TAZ. The stabilized YAP/TAZ in nuclei interacts with TEAD and enhances the transcription of target genes related to anti-apoptosis and proliferation. (B) When Hippo signaling is On (in high cell density): Activation of KIBRA and NF2 via unknown upstream signaling leads to activation of MST1/2. Activated MST1/2 phosphorylate SAV1 which in turn phosphorylate LATS and MOB1. The activated LATS/MOB phosphorylates YAP/TAZ which results in cytoplasmic retention by 14-3-3 protein and proteasomal degradation of YAP/TAZ. As a result, TEAD interacts with VGL4 and suppresses the expression of target genes.
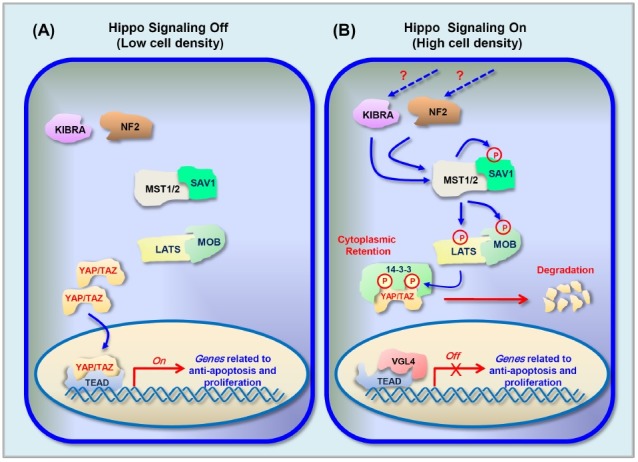
Hippo signaling is regulated in a cell-density-dependent manner. When the MST1/2 and LATS1/2 kinases are activated at high cell density, Hippo signaling is considered to be in the active state. Although the biochemical mechanism is not yet known, MST1/2 are phosphorylated and activated in response to upstream signals (30, 34). The activated MST1/2, in complex with SAV1, activates LATS1/2 and MOB1 by phosphorylation, which in turn phosphorylate the major targets of the Hippo pathway: YAP,at Ser127, and TAZ, at Ser89 (34). The phosphorylated YAP and TAZ are then translocated to the cytoplasm. As a result, the expression of genes mediated by YAP/TAZ and TEAD ceases, and the YAP/TAZ is sequestered by 14-3-3 protein and degraded via the ubiquitin/proteosomal pathway (34, 35).
It has also been shown that the PPxY motifs of Ex (Expanded), Wart, and Hippo directly bind to the WW domain of Yorkie, sequestrating Yorkie to the cytoplasm in a Warts-mediated phosphorylation- independent manner, to inhibit Yorkie activity in Drosophila (36, 37); however, the PPxP motifs of Ex and Hippo necessary for the interaction with Yorkie are not present in mammalian homologs. Thus, a similar phosphorylation-independent mechanism may not be present in the mammalian Hippo pathway.
At low cell density, unphosphorylated YAP/TAZ localizes to the nucleus where they interact with the TEAD group of DNA-binding transcription factors, generally inducing genes involved in anti-apoptosis (such as cell death inhibitor diap1 (38) and the microRNA bantam (39, 40) and pro-proliferation (such as cyclin E (41), E2F1 (42), and dMyc (43)). It should be noted, however, that YAP can function in a cell-context dependent manner. In some cells, the nuclear accumulation of YAP causes apoptosis via the p73-dependent transcription of pro-apoptotic genes.
The upstream regulators of core kinase cascade
The Hippo kinase cascade seems to be controlled by a myriad of upstream signals. Recent studies have shown the apical membrane of epithelial cells as well as junctions at the apical membrane to control the activity of Hippo signaling (3). In both Drosophila and mammalian polarized epithelial cells, adherens junctions separate the apical from the basolateral cell surfaces. Most upstream regulators of Hippo signaling are localized at the apical or subapical membranes as protein complexes. Crumbs, a homophilic cell-cell adhesion molecule at the apical membrane, plays a critical role in the activation of the Hippo pathway via control of the apical localization of Ex. Deletion of Crumbs results in the mis-localization of Ex, which hinders activation of the Hippo pathway. At the subapical membrane, the FERM domain-containing proteins Mer (Merlin) and Ex form a complex with the WW domain-containing protein Kibra to activate Hippo signaling. Although the molecular mechanisms for the activation of Hippo signalingare not yet fully understood, it seems that the Mer-Ex-Kibra complex recruits the members of the core kinase cascade of the Hippo pathway in close proximity (44). This possibility is supported by the fact that Ex binds Hippo, Mer binds Salavdor, and Kibra binds Hippo, Salvador and Warts. Characterization of the upstream signals and molecular mechanisms involved in the activation of the core kinase cascade is one of the top priority subjects in this field. A detailed description of the upstream signals and components can be found in excellent reviews elsewhere (3, 45).
CROSS-TALK BETWEEN WNT AND HIPPO SIGNALING PATHWAYS
The first evidence of cross-talk between the Wnt and Hippo signaling pathway was suggested in 2010 (10). Varelas et al. showed that TAZ blocks the interaction of Ck1δ/ε to Dvl, inhibiting the Wnt3a-induced phosphorylation of Dvl2, and thereby inhibiting Wnt/β-catenin signaling. They also demonstrated ectopic expression of MST or LATS, which leads to cytoplasmic localization of TAZ, to inhibit Wnt3a-mediated reporter activity. However, whether or not endogenous Wnt/β-catenin signaling is inhibited by the activation of the Hippo signaling pathway at the endogenous level still needs to be examined. The fact that the kidneys of TAZ conditional knockout mice showed very little increase of nuclear β-catenin raised questions about the functional relevance of their model in in vivo situations (46).
Moreover, an inhibitory role of Hippo signaling in the Wnt/β-catenin signaling pathway in developing heart was suggested by heart-specific Salvador knockout mice (SAV cKO) (47). Increased heart size as well as increased cardiomyocyte proliferation and nuclear β-catenin was found in SAV cKO (47). In microarray analysis on SAV cKO, it was also discovered that the expressions of important genes in heart development or anti-apoptosis were increased, and that the expressions of these genes were dependent on β-catenin. Finally, results from ChIP and reporter assay suggested that YAP-TEAD and β-catenin-TCF/Lef act cooperatively to induce some of their target genes, sox2 and snail2, when SAV is deleted in developing heart. However, it is still unknown whether similar results would be obtained when other Hippo core components, such as MST and LATS, are conditionally deleted, and whether this inhibitory role of SAV is applicable to all tissues rather than being restricted to the heart.
Later, an additional cross-talk mechanism was suggested (48). Imajo et al. found that YAP2 strongly blocks the constitutively active form of β-catenin (β-catenin S33Y, S37A)-mediated Topflash activity. This would be unexplainable if the inhibitory mechanism of Hippo signaling on Wnt signaling were restricted to the inhibition of Dvl, as constitutively active β-catenin is independent from Dvl activation status. YAP and TAZ interact with β-catenin to block its nuclear localization, inhibiting Wnt/β-catenin signaling. The E66A mutant of YAP, which does not bind to β-catenin, showed much reduced inhibitory activity on Wnt/β-catenin signaling. YAP(S127D), which mimics the phosphorylated-form of YAP and is mainly localized in the cytoplasm, but not YAP(S127A), which mimics the unphosphorylated-form of YAP and is mainly localized in the nucleus, inhibits the nuclear localization of β-catenin. These results suggested that YAP or TAZ inhibit Wnt signaling in two ways: by blocking the activation of Dvl and/or the cytoplasmic sequestration of β-catenin (48).
So far, we have described how Hippo signaling inhibits Wnt/β-catenin signaling. However, Piccolo and colleagues identified a mechanism by which the level of YAP/TAZ is regulated by β-catenin (11, 12). It is a truly provocative idea that TAZ is an integral mediator of Wnt/β-catenin signaling. It was shown that the level of TAZ is regulated in the β-catenin destruction complex, which is composed of APC, Axin, and GSK3β. In the absence of Wnt signaling, β-catenin phosphorylated by GSK3β serves as a scaffold for the association of TAZ with the β-TrCP E3 ligase complex. However, in the presence of Wnt, blocking the phosphorylation of β-catenin leads to escape of both β-catenin and TAZ from the destruction complex, subsequently enhancing expression of the target genes mediated by the β-catenin/Tcf and TAZ/TEAD complexes. Extended from these results, the same group showed YAP/TAZ to be essential for β-TrCP recruitment to the β-catenin destruction complex and subsequent β-catenin degradation. Overall, these data suggest that the Hippo signaling transcription factors YAP and TAZ are integral components of the β-catenin destruction complex, orchestrating the Wnt responses.
CONCLUSION
It is just the beginning to understand how animals control their organ size. Hippo signaling and cross-talk of Hippo and other signaling pathways including Wnt, Shh, and BMP/TGFβ signaling is known to be involved in the regulation of cell growth, proliferation, and death. Since Hippo signaling is a new concept compared to the other well-defined signaling pathways, the identification and characterization of more Hippo signaling components is expected. More specifically, excavation of the upstream regulators of the Hippo signaling pathway and discovery of mechanisms for the regulation of the core kinase cascade are imminent. Discovery of the cross-talk between Hippo and other well-known signaling pathways will deepen our understanding of signaling pathways as a whole network, which may lead to the development of valuable therapeutic targets for curing human diseases such as cancer.
Acknowledgments
This work was supported by the 2013 Research Fund of the University of Seoul.
References
- 1.Pan D. The hippo signaling pathway in development and cancer. Dev. Cell. (2010);19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan D. Hippo signaling in organ size control. Genes Dev. (2007);21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 3.Enderle L., McNeill H. Hippo gains weight: added insights and complexity to pathway control. Sci. Signal. (2013);6:re7. doi: 10.1126/scisignal.2004208. [DOI] [PubMed] [Google Scholar]
- 4.Harvey K. F., Zhang X., Thomas D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer. (2013);13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 5.Yu F. X., Guan K. L. The Hippo pathway: regulators and regulations. Genes Dev. (2013);27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y., Yang Y., Wang F., Wei Q., Qin H. Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int. J. Cancer. (2014) doi: 10.1002/ijc.29073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. (2012);149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Kim W., Kim M., Jho E. H. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem. J. (2013);450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 9.Massague J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. (2012);13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varelas X., Miller B. W., Sopko R., Song S., Gregorieff A., Fellouse F. A., Sakuma R., Pawson T., Hunziker W., McNeill H., Wrana J. L., Attisano L. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell. (2010);18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. (2012);151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., Fassina A., Cordenonsi M., Piccolo S. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. (2014);158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez L. A., Northcott P. A., Dalton J., Fraga C., Ellison D., Angers S., Taylor M. D., Kenney A. M. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. (2009);23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii M., Toyoda T., Nakanishi H., Yatabe Y., Sato A., Matsudaira Y., Ito H., Murakami H., Kondo Y., Kondo E., Hida T., Tsujimura T., Osada H., Sekido Y. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J. Exp. Med. (2012);209:479–494. doi: 10.1084/jem.20111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attisano L., Wrana J. L. Signal integration in TGF-beta, WNT, and Hippo pathways. F1000Prime Rep. (2013);5:17. doi: 10.12703/P5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyer T. A., Weiss A., Khomchuk Y., Huang K., Ogunjimi A. A., Varelas X., Wrana J. L. Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. (2013);5:1611–1624. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., Zhao J., Yuan H., Tumaneng K., Li H., Fu X. D., Mills G. B., Guan K. L. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. (2012);150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regimbald-Dumas Y., He X. Wnt signalling: What The X@# is WTX? EMBO J. (2011);30:1415–1417. doi: 10.1038/emboj.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald B. T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. (2009);17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Orte E., Saenz-Narciso B., Moreno S., Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends. Genet. (2013);29:545–553. doi: 10.1016/j.tig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Sugimura R., He X. C., Venkatraman A., Arai F., Box A., Semerad C., Haug J. S., Peng L., Zhong X. B., Suda T., Li L. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. (2012);150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimura R., Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res. C. Embryo Today. (2010);90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol. Cancer Ther. (2009);8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 24.Willert K., Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. (2012);4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R.,, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. (2003);423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 26.Kim S. E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M. V., MacDonald B. T., Zhang X., Garcia Abreu J., Peng L., He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. (2013);340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li V. S., Ng S. S., Boersema P. J., Low T. Y., Karthaus W. R., Gerlach J. P., Mohammed S., Heck A. J., Maurice M. M., Mahmoudi T., Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. (2012);149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Moya I. M., Halder G. Discovering the Hippo pathway protein-protein interactome. Cell Res. (2014);24:137–138. doi: 10.1038/cr.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson R., Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug. Discov. (2014);13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., Rossant J., Wrana J. L. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell. (2010);19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Yu J., Zheng Y., Dong J., Klusza S., Deng W. M., Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell. (2010);18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin F., Yu J., Zheng Y., Chen Q., Zhang N., Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. (2013);154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N., Bai H., David K. K., Dong J., Zheng Y., Cai J., Giovannini M., Liu P., Anders R. A., Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell. (2010);19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. (2007);21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF (beta-TRCP). Genes Dev. (2010);24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badouel C., Gardano L., Amin N., Garg A., Rosenfeld R., Le Bihan T., McNeill H. The FERM-domain protein Expanded regulates Hippo pathway activity via direct interactions with the transcriptional activator Yorkie. Dev. Cell. (2009);16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Oh H., Irvine K. D. In vivo analysis of Yorkie phosphorylation sites. Oncogene. (2009);28:1916–1927. doi: 10.1038/onc.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J., Wu S., Barrera J., Matthews K., Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. (2005);122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Thompson B. J., Cohen S. M. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. (2006);126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Nolo R., Morrison C. M., Tao C., Zhang X., Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr. Biol. (2006);16:1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 41.Tapon N., Harvey K. F., Bell D. W., Wahrer D. C., Schiripo T. A., Haber D., Hariharan I. K. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. (2002);110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 42.Nelson M. A., Reynolds S. H., Rao U. N., Goulet A. C., Feng Y., Beas A., Honchak B., Averill J., Lowry D. T., Senft J. R., Jefferson A. M., Johnson R. C., Sargent L. M. Increased gene copy number of the transcription factor E2F1 in malignant melanoma. Cancer Biol. Ther. (2006);5:407–412. doi: 10.4161/cbt.5.4.2512. [DOI] [PubMed] [Google Scholar]
- 43.Neto-Silva R. M., de Beco S., Johnston L. A. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell. (2010);19:507–520. doi: 10.1016/j.devcel.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genevet A., Wehr M. C., Brain R., Thompson B. J., Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell. (2010);18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genevet A., Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem. J. (2011);436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 46.Makita R., Uchijima Y., Nishiyama K., Amano T., Chen Q., Takeuchi T., Mitani A., Nagase T., Yatomi Y., Aburatani H., Nakagawa O., Small E. V., Cobo-Stark P., Igarashi P., Murakami M., Tominaga J., Sato T., Asano T., Kurihara Y., Kurihara H. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Renal. Physiol. (2008);294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 47.Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. (2011);332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imajo M., Miyatake K., Iimura A., Miyamoto A., Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. (2012);31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]


