Abstract
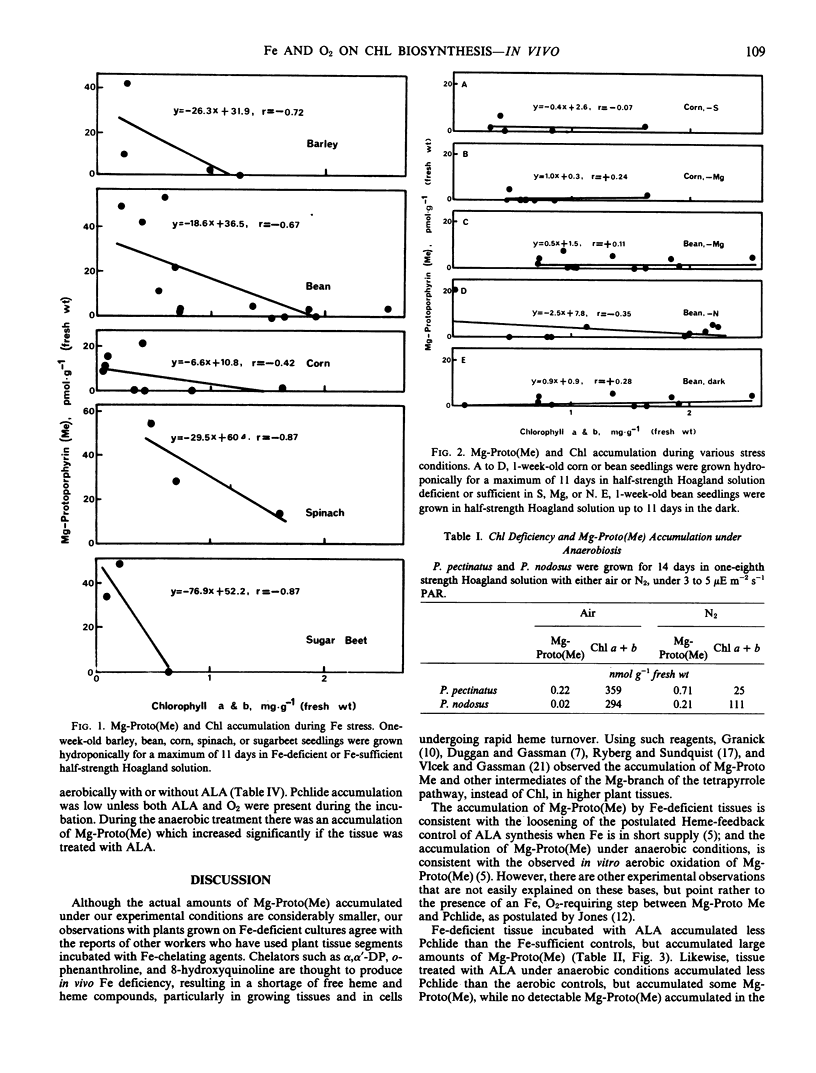
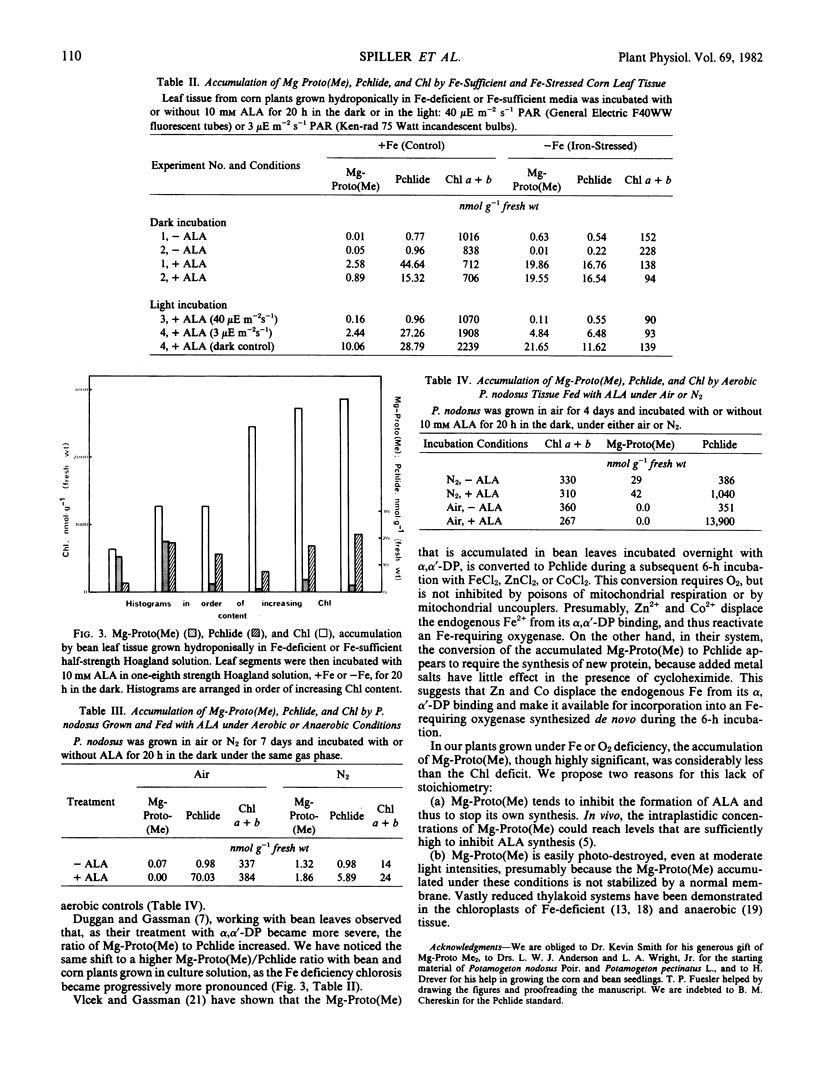
Corn (Zea mays, L.), bean (Phaseolus vulgaris L.), barley (Hordeum vulgare L.), spinach (Spinacia oleracea L.), and sugarbeet (Beta vulgaris L.) grown under iron deficiency, and Potamogeton pectinatus L, and Potamogeton nodosus Poir. grown under oxygen deficiency, contained less chlorophyll than the controls, but accumulated Mg-protoporphyrin IX and/or Mg-protoporphyrin IX monomethyl ester. No significant accumulation of these intermediates was detected in the controls or in the tissue of plants stressed by S, Mg, N deficiency, or by prolonged dark treatment. Treatment of normal plant tissue with δ-aminolevulinic acid in the dark resulted in the accumulation of protochlorophyllide. If this treatment was carried out under conditions of iron or oxygen deficiency, less protochlorophyllide was formed, but a significant amount of Mg-protoporphyrin IX and Mg-protoporphyrin IX monomethyl ester accumulated.
These results are consistent with the presence of an O2, Fe-requiring step between Mg-protoporphyrin IX monomethyl ester and protochlorophyllide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Dekock P. C., Commisiong K., Farmer V. C., Inkson R. H. Interrelationships of Catalase, Peroxidase, Hematin, and Chlorophyll. Plant Physiol. 1960 Sep;35(5):599–604. doi: 10.1104/pp.35.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- Hsu W. P., Miller G. W. Coproporphyrinogenase in tobacco (Nicotiana tabacum L.). Biochem J. 1970 Apr;117(2):215–220. doi: 10.1042/bj1170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Evans H. J., Matrone G. Investigations of the Role of Iron in Chlorophyll Metabolism. II. Effect of Iron Deficiency on Chlorophyll Synthesis. Plant Physiol. 1963 Nov;38(6):638–642. doi: 10.1104/pp.38.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Haidar M. A., Yaghi M. Porphyrin Biosynthesis in Cell-free Homogenates from Higher Plants. Plant Physiol. 1970 Oct;46(4):543–549. doi: 10.1104/pp.46.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek L. M., Gassman M. L. Reversal of alpha,alpha'-Dipyridyl-induced Porphyrin Synthesis in Etiolated and Greening Red Kidney Bean Leaves. Plant Physiol. 1979 Sep;64(3):393–397. doi: 10.1104/pp.64.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Y. Photoactivation of Chlorophyll Synthesis and Cytochrome Oxidase Activity in Anaerobically Germinated Seedlings of Echinochloa crusgalli var. Oryzicola. Plant Physiol. 1980 Mar;65(3):451–454. doi: 10.1104/pp.65.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



