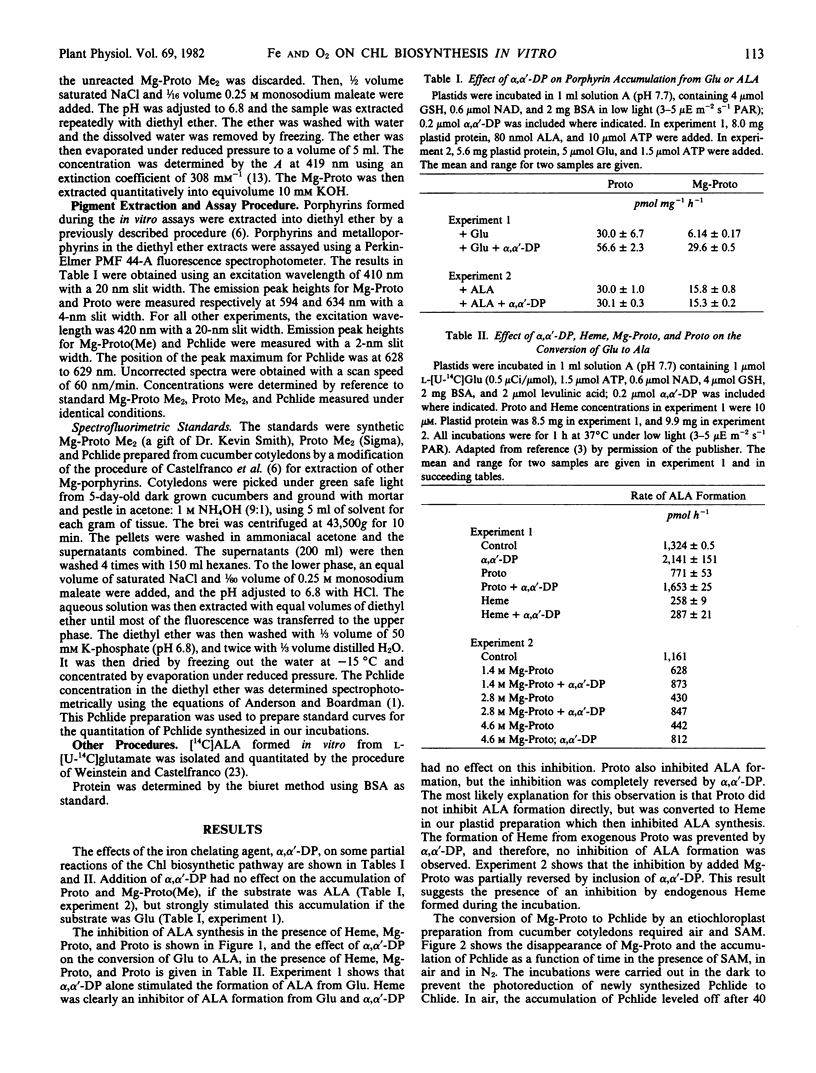
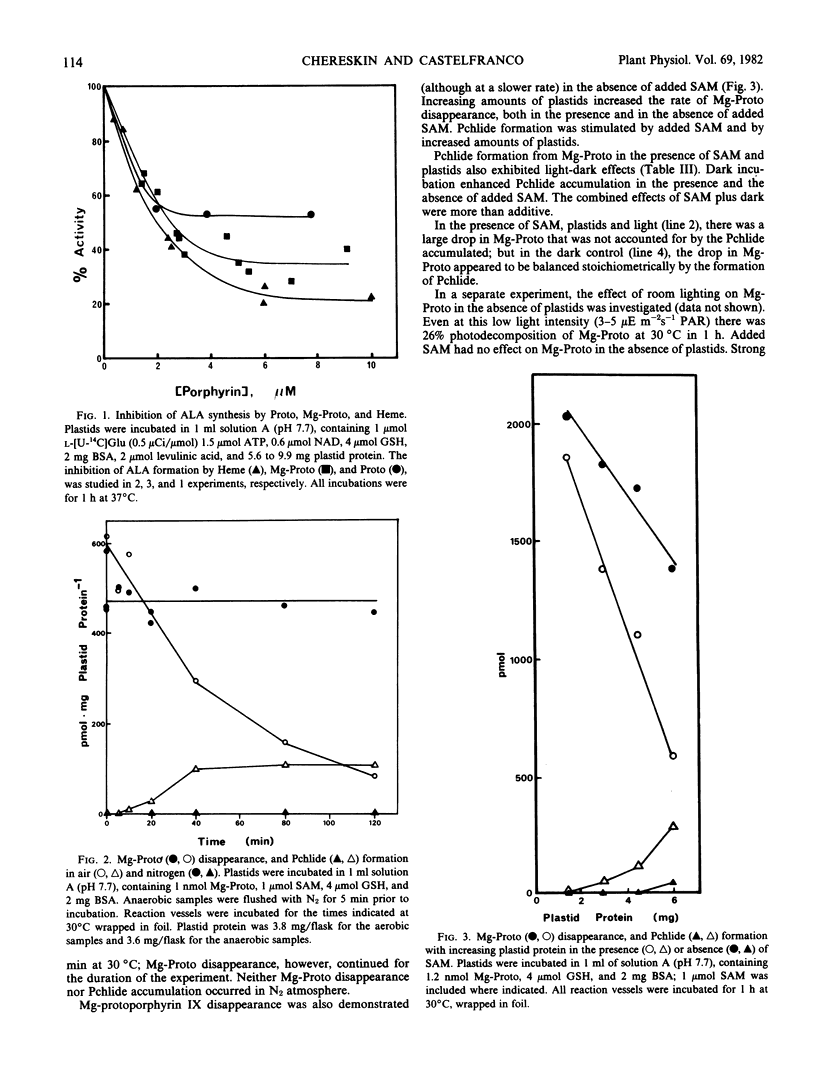
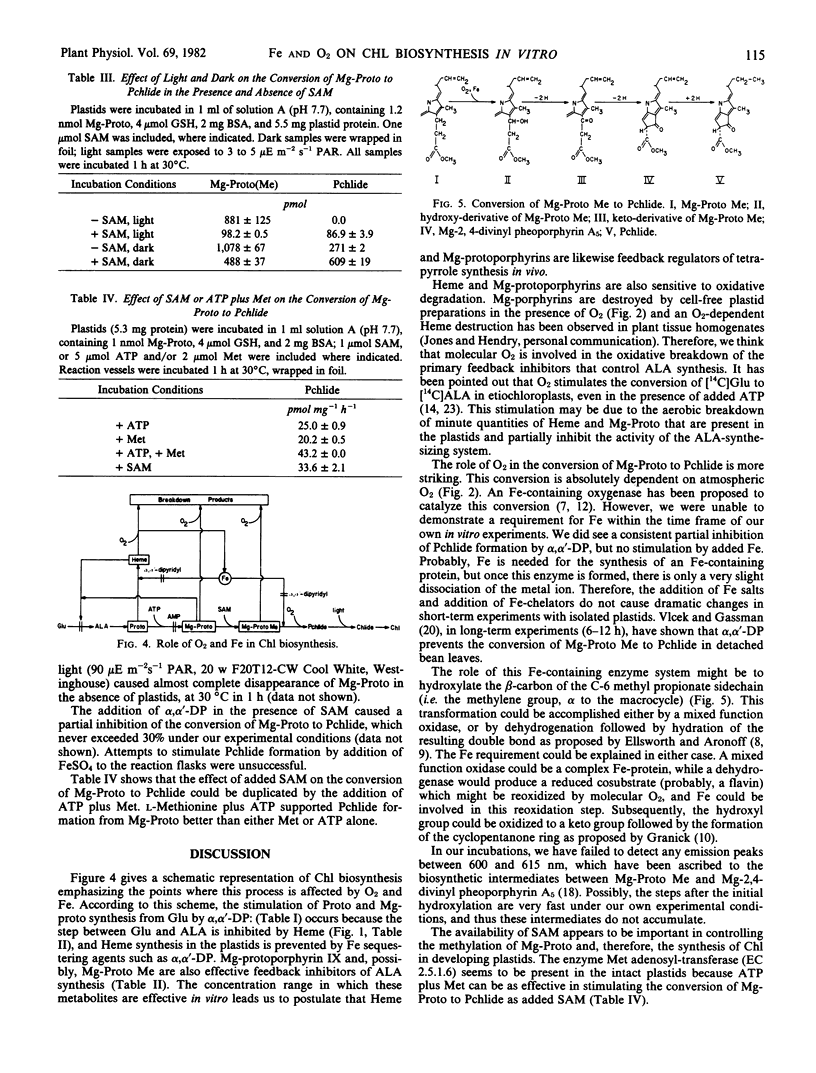
Abstract
The conversion of l-glutamate to δ-aminolevulinate, in preparations of cucumber etiochloroplasts incubated in vitro, was inhibited by protoheme IX and Mg-protoporphyrin IX. Mg-protoporphyrin IX was destroyed in the presence of air and plastids; this breakdown was accelerated by S-adenosyl methionine. Mg-protoporphyrin IX was also converted to protochlorophyllide in vitro. This conversion exhibited an absolute requirement for atmospheric oxygen and was strongly stimulated by S-adenosyl methionine and by darkness.
Based on these results, and on the results of the preceding paper (Spiller, Castelfranco, Castelfranco 1981 Plant Physiol 68: 107-111), a comprehensive hypothesis for the role of O2 and Fe in chlorophyll biosynthesis is formulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Schwarcz S. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. Mg-protoporphyrin-IX synthesis. Arch Biochem Biophys. 1978 Mar;186(2):365–375. doi: 10.1016/0003-9861(78)90447-2. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations of the biogenesis of chlorophyll a. IV. Isolation and partial characterization of some biosynthetic intermediates between Mg-protoporphine IX monomethyl ester and Mg-vinylpheoporphine a5, obtained from Chlorella mutants. Arch Biochem Biophys. 1969 Mar;130(1):374–383. doi: 10.1016/0003-9861(69)90047-2. [DOI] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations on the biogenesis of chlorophyll a. 3. Biosynthesis of Mg-vinylpheoporphine a5 methylester from Mg-protoporphine IX monomethylester as observed in Chlorella mutants. Arch Biochem Biophys. 1968 Apr;125(1):269–277. doi: 10.1016/0003-9861(68)90661-9. [DOI] [PubMed] [Google Scholar]
- GRANICK S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948 1949;Series 44:220–245. [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. The production of magnesium protoporphyrin monomethyl ester by Rhodopseudomonas spheroides. Biochem J. 1963 Mar;86:429–432. doi: 10.1042/bj0860429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little H. N., Jones O. T. The subcellular loclization and properties of the ferrochelatase of etiolated barley. Biochem J. 1976 May 15;156(2):309–314. doi: 10.1042/bj1560309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S., Hehlein C., Rüdiger W. Influence of Anaerobiosis on Chlorophyll Biosynthesis in Greening Oat Seedlings (Avena sativa L.). Plant Physiol. 1980 Oct;66(4):576–579. doi: 10.1104/pp.66.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. B., Rebeiz C. A. Chloroplast biogenesis: detection of Mg-protoporphyrin chelatase in vitro. Arch Biochem Biophys. 1977 Apr 15;180(1):178–185. doi: 10.1016/0003-9861(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Thomas J., Ross C. W., Chastain C. J., Koomanoff N., Hendrix J. E. Cytokinin-induced wall extensibility in excised cotyledons of radish and cucumber. Plant Physiol. 1981 Jul;68(1):107–110. doi: 10.1104/pp.68.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek L. M., Gassman M. L. Reversal of alpha,alpha'-Dipyridyl-induced Porphyrin Synthesis in Etiolated and Greening Red Kidney Bean Leaves. Plant Physiol. 1979 Sep;64(3):393–397. doi: 10.1104/pp.64.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Castelfranco P. A. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. delta-Aminolevulinic acid sysnthesis. Arch Biochem Biophys. 1978 Mar;186(2):376–382. doi: 10.1016/0003-9861(78)90448-4. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Castelfranco P. A. Protoporphyrin IX biosynthesis from glutamate in isolated greening chloroplasts. Arch Biochem Biophys. 1977 Jan 30;178(2):671–673. doi: 10.1016/0003-9861(77)90239-9. [DOI] [PubMed] [Google Scholar]


