Abstract
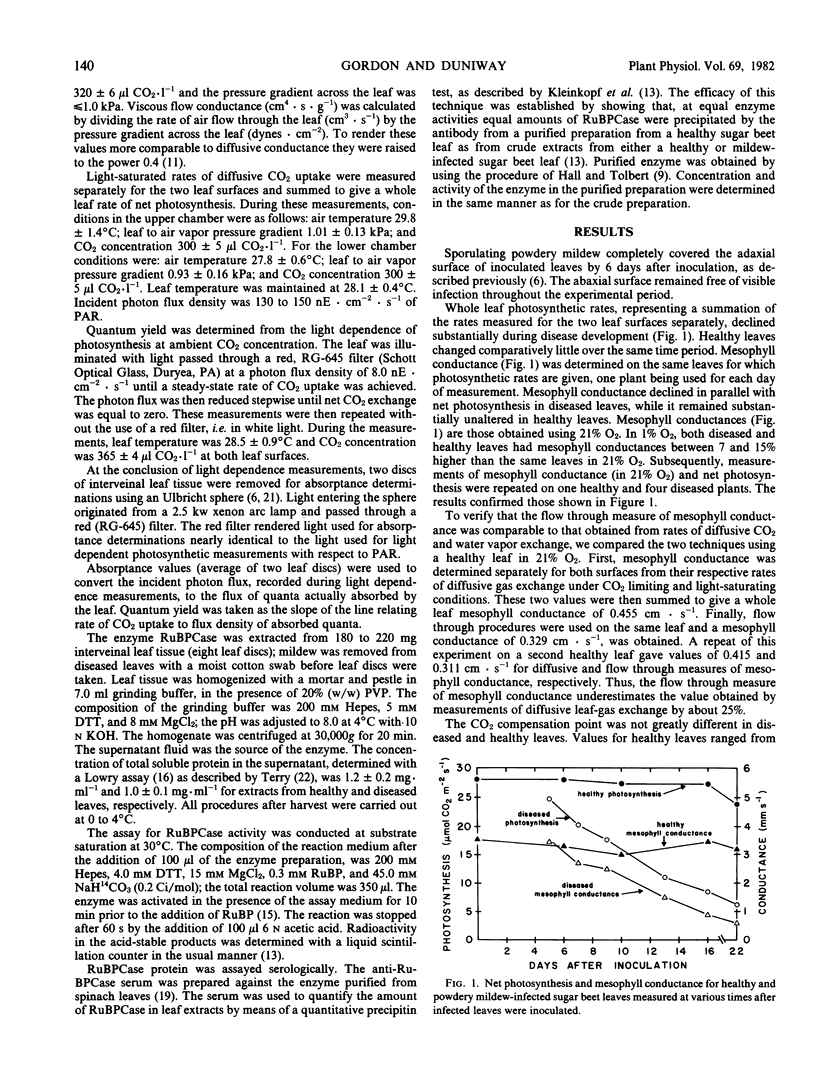
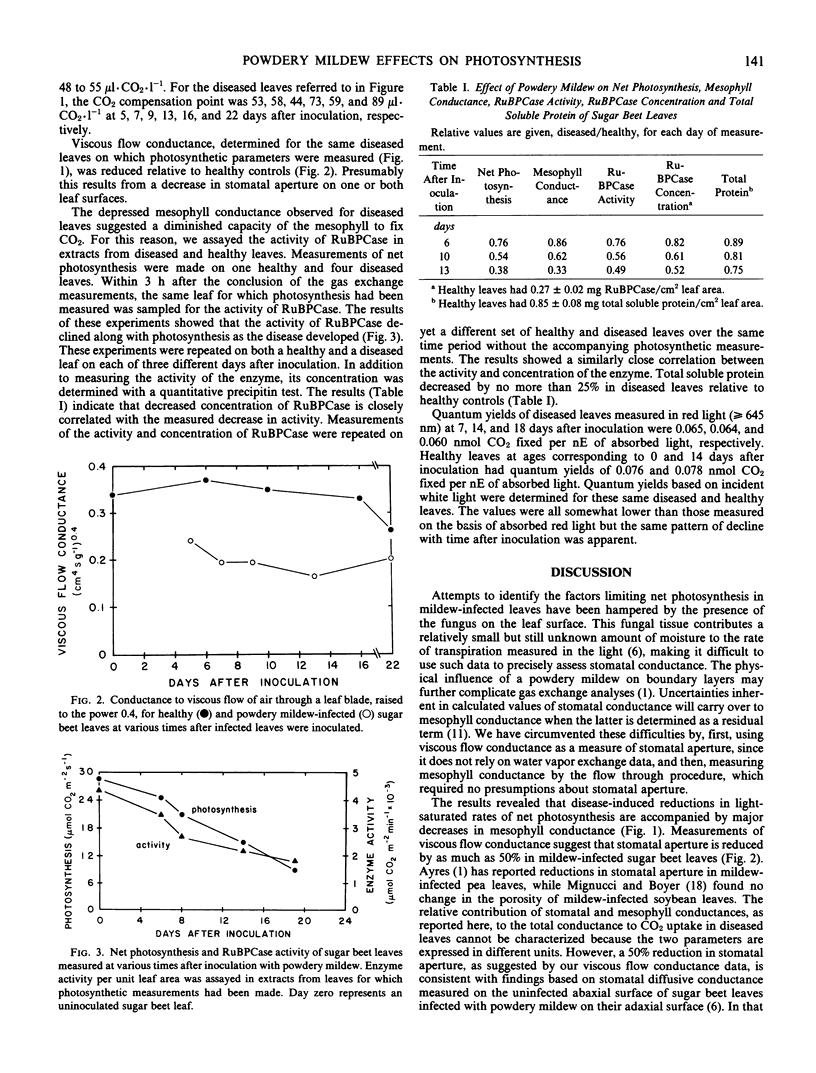
Sugar beet leaves (Beta vulgaris L.) infected with powdery mildew (Erysiphe polygoni D.C.) show declining rates of net photosynthesis as the disease develops; relative to healthy controls, reductions of 35, 70, and 75% were observed at 9, 16, and 22 days after inoculation, respectively. A leaf gas exchange procedure in which an air stream flowed through the leaf showed that mesophyll conductance declined in parallel with photosynthesis in mildew-infected leaves. Viscous flow conductance of diseased leaves also declined over the same period suggesting that stomatal aperture was reduced. From the magnitude and time course of disease effects on stomatal aperture and mesophyll conductance, it appears that the effects at the mesophyll level were primarily responsible for mediating the decline in net photosynthesis. Changes in mesophyll conductance were closely correlated with reduced activity of ribulose-1,5-bisphosphate carboxylase on a leaf area basis. This decrease could be attributed to a reduction in the concentration of the enzyme, a reduction which was greater than the reduction in total soluble protein. The quantum efficiency of light use was also decreased by the disease. Mildew-infected leaves had quantum yields that were reduced, relative to healthy leaves, by 17 and 22% at 14 and 18 days after inoculation, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards H. H. Biphasic inhibition of photosynthesis in powdery mildewed barley. Plant Physiol. 1970 May;45(5):594–597. doi: 10.1104/pp.45.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. E., Loomis R. S. An Explanation for the Difference in Photosynthetic Capabilities of Healthy and Beet Yellows Virus-infected Sugar Beets (Beta vulgaris L.). Plant Physiol. 1972 Nov;50(5):576–580. doi: 10.1104/pp.50.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Magyarosy A. C., Schürmann P., Buchanan B. B. Effect of powdery mildew infection on photosynthesis by leaves and chloroplasts of sugar beets. Plant Physiol. 1976 Apr;57(4):486–489. doi: 10.1104/pp.57.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H., Stoddart J. L. Separation of Chlorophyll Degradation from Other Senescence Processes in Leaves of a Mutant Genotype of Meadow Fescue (Festuca pratensis L.). Plant Physiol. 1975 Sep;56(3):438–441. doi: 10.1104/pp.56.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]


