Abstract
Objective
To evaluate the efficacy and toxicity of carboplatin, granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant interferon gamma 1b (rIFN-γ1b) in women with recurrent, platinum-sensitive ovarian, fallopian tube and primary peritoneal cancer.
Methods
In this phase II study, patients with recurrent, platinum-sensitive ovarian, fallopian tube or primary peritoneal cancer were treated with subcutaneous GM-CSF and rIFN-γ1b before and after intravenous carboplatin until disease progression or unacceptable toxicity. All patients had measurable disease and a chemotherapy-free interval ≥6 months. Response was determined using RECIST criteria and CA 125 levels.
Results
Between 2003 and 2007, 59 patients received a median of 6 cycles of therapy (range, 1 to 13 cycles). Median age at enrollment was 61 years (range, 35 to 79 years). Median time to progression prior to enrollment was 11 months (range, 6 to 58 months). Of 54 patients evaluable for response, 9 (17%) had a complete response, 21 (39%) had a partial response, and 24 (44%) had progressive disease. The overall response rate was 56% (95% CI: 41% to 69%). With a median follow-up of 6.4 months, median time to progression was 6 months. Myeloid derived cells and platelets increased on day 9 of each chemotherapy cycle. The most common adverse effects were bone marrow suppression, carboplatin hypersensitivity, and fatigue. Responders reported improved quality of life.
Conclusion
This pre and post-carboplatin cytokine regimen resulted in a reasonable response and a hematologic profile that could invite further evaluation of its components in the treatment of patients with ovarian cancer.
Keywords: ovarian cancer, carboplatin, immunotherapy, GM-CSF, interferon
Introduction
Ovarian cancer patients who initially respond to platinum-based chemotherapy and maintain a disease-free interval of ≥6 months are considered to have potentially platinum-sensitive disease [1]. Carboplatin remains the cornerstone of treatment for such patients due to its favorable therapeutic profile. The addition of agents including paclitaxel [2, 3], gemcitabine [4], docetaxel [5] and pegylated liposomal doxorubicin [6] have shown improved response and progression-free survival rates, though with overall higher toxicity. Recurrent and persistent ovarian cancer remains a target for treatment developments that can contribute to improved patient outcomes.
Monocytes and macrophages (MO/MAs) are innate immune cells that mediate both antibody-dependent and independent tumor-cell cytotoxicity in-vitro. MO/MA mediated immune anti-tumor activity can be stimulated by either granulocyte-macrophage colony-stimulating factor (GM-CSF) or recombinant interferon gamma 1b (rIFN- γ1b) [7-9]. GM-CSF has not previously been studied in a phase II adjuvant setting for ovarian cancer. However, the results of two non-randomized trials in melanoma [10] and breast cancer [11] suggest that adjuvant GM-CSF may contribute to improved survival.
Previous studies have shown that rIFN- γ1b has clinical activity in ovarian cancer [12-14]. A report by Pujade-Lauraine et al. [12] showed an overall response rate of 31% in 108 patients with residual disease at second-look laparotomy treated with intraperitoneal rIFN- γ1b. In addition, a phase III study showed an increase in PFS from 38 to 51% when rIFN- γ1b was added to cisplatin and cyclophosphamide as primary treatment for ovarian cancer [13]. A subsequent phase I/II study combining rIFN- γ1b with paclitaxel and carboplatin reported a 72% response rate [14]. However, following completion of the current trial, a phase III study in advanced ovarian cancer was published that showed a shorter survival in patients treated with carboplatin, paclitaxel and rIFN-γ1b compared with carboplatin and paclitaxel alone [15]. In addition, serious adverse events were more common in the group receiving rIFN-γ1b, primarily due to a higher incidence of serious hematological toxicities.
We conducted a phase II study of GM-CSF and rIFN-γ1b administered before and after carboplatin in patients with recurrent, platinum-sensitive ovarian cancer. The study was modeled in part on a previous report of intravenous GM-CSF given before and after a combination chemotherapy regimen in patients with metatstatic sarcoma [16]. This resulted in increased myeloid cellularity with increased blood neutrophil and monocyte levels and a reduced duration of neutropenia. In the current trial, a more patient convenient regimen of daily subcutaneous injections of GM-CSF was utilized, with rIFN-γ1b administered during the latter part of the GM-CSF cycle to enhance MO/MA mediated cytotoxicity.
The primary objectives of the study were to evaluate the response and toxicity of this treatment regimen. Secondary objectives included evaluating time to progression (TTP) and effects on quality of life (QOL). In addition to evaluating hematologic responses, an in-vitro model assay was utilized to measure antibody-dependent cell mediated cytotoxicity (ADCC) against a defined antigen.
Materials and Methods
Patients
Eligible patients included women with recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer who had initially responded to first-line, platinum-based chemotherapy with a time to progression ≥6 months. Patients were required to have measurable disease; Zubrod performance status score ≤2; and adequate hematologic, renal and hepatic function. Ineligibility criteria included >2 prior chemotherapy regimens; prior immunotherapy; prior abdominal radiotherapy; active heart, autoimmune or inflammatory bowel disease; brain metastases; albumin ≤3 g/dL; and prior hypersensitivity to platinum agents. The Institutional Review Board approved the study, and all patients provided informed consent prior to participation.
Procedures
Each cycle of treatment comprised subcutaneous injections of GM-CSF (Leukine®, Bayer HealthCare Pharmaceuticals, Inc., Seattle, WA) in two 7-day courses, one preceding and one following a fixed intravenous dose of carboplatin (Paraplatin®, Bristol-Myers Squibb, New York, NY), at an area under the curve (AUC) of 5 mg/mL/min using the Calvert formula [17]. Carboplatin was administered 48-60 hours following the last dose of GM-CSF. Post-chemotherapy, GM-CSF was started within 24-36 hours of the carboplatin infusion. For the first cycle of treatment, GM-CSF was administered at 400 mcg daily. If the patient's monocyte level did not both double and increase to ≥1000 cells/mL by day 9 after carboplatin, the dose was escalated to 600 mcg for the second carboplatin dose and for all subsequent cycles. rIFN-γ1b (Actimmune®, InterMune, Brisbane, CA) was administered by a subcutaneous injection at a fixed dose of 100 mcg on the fifth and seventh day of each 7-day cycle of GM-CSF. For subsequent courses, the pre-chemotherapy GM-CSF/rIFN-γ1b cycle started 20 days following the previous carboplatin administration. Treatment was continued until disease progression or unacceptable toxicity.
Baseline assessments included medical history; physical examination; complete blood count (CBC); blood chemistry analysis including renal and hepatic function; and CA 125 level. To monitor the effects of GM-CSF and rIFN-γ1b, a CBC was also obtained on days -4, 1, 6,9,16, 21, 25 and 28 of the first cycle and on days 1, 9, 16, 21 and 28 of the second and subsequent cycles. If the ANC was ≤1,000 cells/mL, or platelet count <100,000 cells/mL, subsequent treatment was delayed until recovery, and the second rIFN-γ1b dose discontinued for each subsequent GM-CSF cycle. Patients were removed from the study if the toxicities did not resolve after a two-week treatment delay. Chest radiograph and computed tomography (CT) scan of the abdomen/pelvis were obtained prior to enrollment and after every 3 cycles of treatment.
Response
All patients who received at least one cycle of treatment, a tumor evaluation at baseline, and at least one subsequent evaluation were evaluable for response using the modified World Health Organization Response Evaluation Criteria in Solid Tumors (RECIST) [18]. Complete response (CR) was defined as disappearance of all lesions and normalization of CA 125 levels. Partial response (PR) was defined as ≥30% decrease in target lesions, without progression of non-target lesions or development of new lesions. In addition to RECIST criteria, a PR was considered if CA 125 levels declined by ≥50%, provided target lesion size did not increase by ≥20% on imaging [19, 20]. Progressive disease (PD) was defined as ≥20% increase in target lesion, progression of existing non-target lesions, or appearance of new lesions. Stable disease (SD) was an insufficient decrease in tumor size to qualify as a PR or an insufficient increase in tumor size to qualify as PD. All response evaluations were performed ≥4 weeks apart. In the absence of a rise in CA 125, responses were confirmed radiologically after every 3 cycles.
Toxicity
Toxicity was assessed at every cycle and graded using the U.S. National Cancer Institute's Common Toxicity Criteria [21]. All patients undergoing at least one treatment cycle were evaluable for toxicity. Any toxicity needed to return to ≤grade 1 in order to continue treatment. Patients who developed carboplatin hypersensitivity were treated with appropriate desensitization. Patients with recurrent grade 3/4 toxicities were removed from the study.
Quality of Life
QOL was assessed using the Functional Assessment of Chronic Illness Therapy – Biologic Response Modifier (FACT-BRM version 4) [22], a cancer-specific measure of health-related QOL. It consists of 27 items assessing physical, social and family, emotional and functional well-being (FACT-G) [23] and 17 items assessing issues specific to receiving cytokine therapy (BRM) [24]. Higher scores represent better QOL. Recent research suggests a change in total score of ≥7 on the FACT-G indicates a clinically significant change in QOL [25]. Questionnaires were completed on days -8, -4, -1 and 9 during the first treatment cycle, and on days -1 and 9 for all subsequent cycles.
Cell-Mediated and Antibody-Dependent Cellular Toxicity
Peripheral blood specimens were collected day -8, -4, 1 and 9 of cycle 1 of chemotherapy in patients consenting to optional studies. Purified monocytes were tested for their capacity to kill tumor cells both with and without an antibody capable of mediating ADCC using a previously described 3H-TdR release assay [26, 27]. The antibody construct employed in this assay was obtained from Macrogenics, Rockville, MD [28] and targets the HER2/neu antigen which is highly expressed on SKOV3 tumor cells. HER2/neu was used as a model antigen for ADCC.
Briefly, purified monocytes were isolated from patient blood mononuclear cells or buffy coat by negative selection, using a Monocyte Isolation Kit, and a MACS separator (MIltenyi Biotec, Inc., Auburn CA). Labeled HER2/neu SKOV3 (human ovarian cancer cells, ATCC HTB-77, Manassas, VA) were incubated with Ch4D5WT (chimeric version of HER-2/neu antibody Ch4D5, Ch4D5-N297Q (deglycosylated analogue antibody as a control) at a concentration 10 mcg/105 tumor cells and a media without antibody (control) for 30 minutes at 37 degrees Celsius in a shaking water bath followed by the addition of isolated monocytes.
Statistical Analyses
A Simon's two-stage design [29] was used to evaluate response rates. Sample size calculations were based on a response rate of 30% with the standard treatment used at the time of the trial design [30], and a targeted response rate of 45%. Planned accrual was 65 patients, with a minimum of 25 objective responses at the first stage. The study was discontinued after enrollment of 59 patients following completion of the main study objectives.
The response rate was calculated on all enrolled patients evaluable for response. A corresponding 95% confidence interval (95% CI) for the response rate was estimated. TTP was calculated from date of enrollment to date of progression or date of death from any cause. Mixed model analyses were conducted to examine changes in QOL over time, and post-hoc analyses compared values at baseline and 9 days post-carboplatin for each cycle. T-tests were used to compare changes in eosinophil counts between patients with carboplatin hypersensitivity reactions and those without. For the ADCC evaluation, analysis of variance was used to compare the percent of cell lysis between groups. All P values were two-sided, and a value of <0.05 was considered statistically significant. Analyses were performed using SAS (version 9.1) and R (version 2.6.0) software.
Results
Patient Characteristics
Between January 2003 and July 2007, 59 patients were enrolled in the study. Clinical characteristics are summarized in Table 1. Median age at enrollment was 61 years (range 35 to 79 years). Median chemotherapy-free interval prior to enrollment was 13 months (range 7 to 58 months), with 27 patients (46%) having a chemotherapy free-interval <12 months. Forty-two patients (71%) had received only one prior chemotherapy regimen. Study participants received a median of 6 cycles of therapy (range 1-13 cycles).
Table 1. Patient Characteristics.
| Characteristic | N = 59 patients |
|---|---|
|
| |
| Age at enrollment (years): | |
| Median | 61 |
| Range | 35 – 79 |
|
| |
| Zubrod performance status: | |
| 0 | 54 (92%) |
| 1 | 2 (3%) |
| 2 | 3 (5%) |
|
| |
| Site: | |
| Ovary | 50 (85%) |
| Peritoneum | 6(10%) |
| Fallopian tube | 3 (5%) |
|
| |
| Histologic subtype: | |
| Serous | 49 (83%) |
| Adenocarcinoma. NOS | 5 (8%) |
| Clear cell | 3 (5%) |
| Transitional cell | 1 (2%) |
| Endometrioid | 1 (2%) |
|
| |
| Chemotherapy-free interval (months): | |
| Median (Range) | 13 (7-58) |
| 6-12 months | 27 (46%) |
| 13-24-months | 17(29%) |
| > 24 months | 15(25%) |
|
| |
| Prior chemotherapy regimens: | |
| 1 | 42 (71 %) |
| 2 | 17(29%) |
|
| |
| First-line chemotherapy: | |
| Paclitaxel or docetaxol and carboplatin | 54(91%) |
| Paclitaxel and cisplatlnum | 3 (5%) |
| Cisplatinum and cyclophosphamide +/- doxorubicin | 2 (4%) |
|
| |
| Prior re-induction chemotherapy: | |
| Single-agent carboplatin | 8(14%) |
| Paclitaxel and carboplatin | 5 (8%) |
| High-dose chemotherapy with bone marrow transplant | 2 (3%) |
| Gemcitabine | 1 (2%) |
| Intraperitoneal IL-12 (two doses) | 1 (2%) |
| None | 42 (71 %) |
Response
Of the 54 evaluable patients, 9 (17%) had a CR, 21 (39%) had a PR, and 24 (44%) had PD. The overall response rate was 56% (95% CI: 41 to 69%). For 6 of the 21 patients with a PR (29%), the response was based on CA 125 criteria (>50% decline in CA 125 level, provided target lesion size did not increase by ≥20% on imaging). With a median follow-up of 6.4 months, the median TTP was 6 months.
Toxicity
58 patients received ≥1 cycle of treatment and were evaluable for toxicity (Table 2). Five patients withdrew from the study before they could be evaluated for response due to fatigue (n=2), rash (n=1), carboplatin sensitivity reaction (n=1), and “high-feeling” associated with the treatment (n=1). The most common adverse effects were bone marrow suppression, fatigue and carboplatin hypersensitivity reactions. In 18 patients, the second dose of interferon was dropped due to neutropenia (n=9), thrombocytopenia (n=8), or both (n=1). No patients were removed from the study due to bone marrow suppression. There were no treatment-related deaths.
Table 2. Toxicity (N=58 evaluable patients).
| Toxicity | Grade 3 toxicity | Grade 4 toxicity |
|---|---|---|
|
| ||
| Hematologic | ||
| Neutropenia | 12 (21%) | 4 (7%) |
| Thrombocytopenia | 9 (16%) | 0 (0%) |
| Anemia | 2 (3%) | 0 (0%) |
|
| ||
| Fatigue | 16 (28%) | 4 (7%) |
|
| ||
| Allergic reaction | 15 (26%) | 0 (0%) |
|
| ||
| Myalgia/arthralgias | 10 (17%) | 0 (0%) |
|
| ||
| Depression/anxiety | 5 (9%) | 3 (5%) |
|
| ||
| Nausea/vomiting | 5 (9%) | 0 (0%) |
|
| ||
| Neurotoxicity | 3 (5%) | 0 (0%) |
|
| ||
| Headache | 2 (3%) | 0 (0%) |
|
| ||
| Elevated liver function tests | 2 (3%) | 0 (0%) |
|
| ||
| Elevated creatinine | 0 (0%) | 0 (0%) |
|
| ||
| Alopecia | 0 (0%) | 0 (0%) |
|
| ||
| Fever | 0 (0%) | 0 (0%) |
Fifteen patients (26%) developed a carboplatin hypersensitivity reaction requiring desensitization. The reactions occurred after a median of 2 cycles (range 2 to 7 cycles) with 2 patients unable to continue treatment despite the desensitizing regimen. Subset analysis of the 14 patients who were desensitized and evaluable for response showed 3 patients (21%) with a CR, 5 patients (36%) with a PR and 6 patients (43%) with PD, for a total response rate of 57%.
Quality of Life
QOL questionnaires were complete for 49 patients (83%) during cycle one, 44 patients (75%) during cycle two, 42 patients (71%) during cycle three, 34 patients (58%) during cycle four, 27 patients (46%) during cycle five and 21 patients (36%) during cycle six. QOL data from the remaining cycles were not analyzed as more than 30% of patients had discontinued the study. FACT-G scores 9 days post-carboplatin were lower than baseline scores for each cycle of therapy, with the greatest decrease seen with cycle one (baseline 86.6, cycle one 77.9 (p <.0001), cycle two 79.0 (p<.0003), cycle three 81.2 (p<.01), cycle four 81.4 (p<.03), cycle five 80.9 (p<.03) and cycle six 81.2 (p<.06)). Similar analyses examining BRM scores revealed differences between baseline and day 9 post-carboplatin levels were only statistically significant at cycle 4 (baseline 53.7, cycle one 49.8 (p=.08), cycle two 49.6 (p<.06), cycle three 50.6 (p=.17), cycle four 48.8 (p<.05), cycle five 51.2 (p=.36) and cycle six 56.8 (p=.29)). Pretreament FACT-G scores were not significantly different than baseline levels after the first cycle and for subsequent cycles (baseline 86.6, pre-treatment cycle 1-6 range 83.2-84.5). Pretreatment BRM scores were significantly lower than baseline at the fourth cycle (baseline 53.7, cycle four 48.7, p<.02), but not at cycles 5 and 6. Differences in FACT-G scores were driven by the physical and functional well-being subscales (data not shown).
Patients with a PR at the end of 3 cycles had better FACT-G scores at baseline and throughout treatment than patients with SD or PD (Figure 1); none of the patients had a CR at cycle 3, but all the eventual CRs had a PR at cycle 3. One-way ANOVAS examining group differences at each time point revealed marginally significant differences in FACT-G scores at baseline (P = .054), and significant differences by the end of cycle 2 (P = 0.016) and cycle 3 (P = .003). There were no differences between patient groups for the BRM scores.
Figure 1.

Quality of life scores across three cycles of treatment based on response.
 Partial response
Partial response
 Stable disease
Stable disease
 Progressive disease
Progressive disease
Hematologic and Immunologic Effects
Longitudinal hematologic changes during treatment are shown in Figure 2. Patients developing hypersensitivity were excluded from this analysis because of steroid usage. All values are reported for day 9 of each cycle, based on preliminary results showing maximum increase in MO after GM-CSF at this timepoint [27]. Although, there was mild variability between cycles, the median WBC, neutrophil and monocyte counts peaked at cycle 1 and remained elevated above baseline levels for all 6 cycles. Median platelet counts increased during cycles 2 and 3, and approximated baseline values for the remainder of treatment. No significant differences were observed in monocyte levels on day 9 after chemotherapy cycles 1, 3 and 6 between responding and non-responding patients (data not shown). To determine whether an immunologic cellular response might be involved in patients who developed carboplatin hypersensitivity reactions, mean eosinophil differences in the first 3 cycles were compared between patients with and without reactions, and no significant differences were noted.
Figure 2. Hematologic cell counts.

White blood cell, neutrophil, monocyte and platelet profiles through 6 cycles of chemo-immunotherapy regimen. All values reported as median with 95% confidence intervals.
We first determined whether MO from this study population could function as effector cells, either by direct cytotoxicity (without antibody) or by mediating ADCC, and the possible effects of the cytokines in-vivo (Figure 3). MO exhibited cytotoxicity (mean % cell lysis +/- SD) against the SKOV3 tumor cell targets of 7.2% (+/-3.8%). When HER2/ neu antibody was added, the cytotoxicity increased to 24.4% (+/-8.8%). MO from normal donors showed more direct cytotoxicity than MO from pre-GM-CSF treated patients. The direct MO cytotoxicity increased after GM-CSF, but returned to the pre-treatment baseline after the combination of GM-CSF and rIFN-γ1b. No significant enhancement of ADCC was observed after either GM-CSF alone or GM-CSF plus rIFN-γ1b.
Figure 3. Monocyte/macrophage (MO/MA) and HER2/neu antibody dependent cytotoxicity.
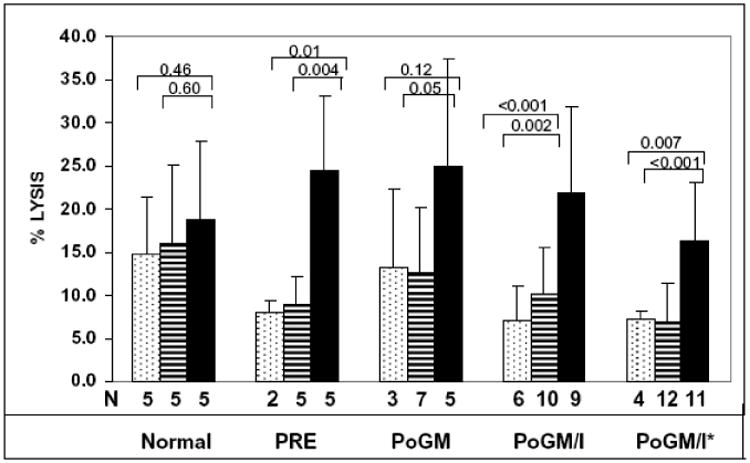
Mean percent lysis of HER2/neu positive SKOV3 ovarian tumor cells in 3H-TdR assay. Vertical coded bars with standard deviation bars represent cytotoxicity results from SKOV3 + MO/MA − antibody
 SK0V3 + MO/MA + N297Qcontrol analogue antibody
SK0V3 + MO/MA + N297Qcontrol analogue antibody
 SKOV3 + MO/MA + WT (anti-HER2/neu monoclonal antibody)
SKOV3 + MO/MA + WT (anti-HER2/neu monoclonal antibody)

N= number of samples tested. Horizontal bars represent p-values calculated for the difference between mean of WT and N297Q and MO/MA control respectively. PRE: pre-treatment; PoGM: post treatment with GM-CSF; PoGM/l: post-treatment with GM-CSF and rlFN-γ1b; PoGM/l*: post-treatment with GM-CSF and rlFN-γ1b after chemotherapy cycle #1.
Discussion
The main goals of therapy in patients with recurrent ovarian cancer are to provide clinical benefit, while limiting toxicity. The current study evaluated the response and toxicity profile of two immunomodulatory cytokines, GM-CSF and rIFN-γ1b, administered before and after carboplatin. Overall response rate was 56% (95% CI: 41 to 69%) with a median TTP of 6 months. The treatment was reasonably well tolerated.
Standard treatment for recurrent disease in platinum-sensitive patients includes re-treatment with single-agent platinum or platinum in combination with other agents [2-6]. In a multivariate analysis of pooled data from 6 phase II trials conducted in 273 platinum-pretreated patients with recurrent ovarian cancer with a chemotherapy-free interval ≥6 months, the response rate to single agent carboplatin was 32.2% [30]. Secondary responses to platinum may increase in relation to chemotherapy-free interval [1]. Twenty-five percent of our patients had a chemotherapy-free interval >24 months, and their response rate was 54%.
In our study, grade 3/4 neutropenia (28%) and thrombocytopenia (16%) were somewhat higher than expected. In this respect, the previous adjuvant rIFN-γ1b study demonstrated higher hematological toxicities in patients receiving rIFN-γ1b in addition to carboplatin and taxol compared with taxol and carboplatin alone (34.5% vs. 22.7%) [15]. Furthermore, in the current study, treatment was usually able to continue after discontinuing the second dose of rIFN-γ1b. By contrast, myeloid derived components including neutrophils, monocytes and eosinophils were significantly above baseline on day 9 following each of six cycles of chemotherapy. Interestingly, platelet levels were increased during cycles 2 and 3 of therapy. No patients were removed from the study due to bone marrow suppression. The mechanisms underlying this platelet response are unknown and may deserve further study since GM-CSF is primarily a myeloid growth factor.
Responses were unaffected in the 15 patients (26%) who experienced carboplatin hypersensitivity reactions, and only two of the patients were unable to continue treatment despite desensitization. This hypersensitivity frequency was comparable to previous reports for carboplatin retreated patients [31-33]. In addition, the recent rIFN-γ1b trial did not show an increase in hypersensitivity when carboplatin and paclitaxel were combined with rIFN-γ1b [15]. Moreover, the current study did not show significant differences in mean eosinophil counts between the patients who experienced a hypersensitivity reaction and those who did not. This does not preclude a possible IgE effect.
Patients reported deterioration in general aspects of QOL while on treatment, most likely due to the added cytokines. However, this effect became less at subsequent cycles, and only a transient decrease in treatment-specific aspects of QOL at cycle 6. There are thus fewer changes in QOL the longer the patients remain on treatment, as the patient with diminishing QOL will come off study. Our findings also suggest that patients who are responding to treatment after three cycles report having better QOL throughout treatment, including at baseline. This suggests that QOL is not diminished by the treatment for patients who are responding and decreases in QOL are also attributed to disease burden and disease progression. Our results offer support for the inclusion of QOL instruments to measure additional endpoints in future trials that incorporate biologic agents for the treatment ovarian cancer.
The effect of adding a monoclonal antibody that targets the HER2/neu molecule was examined in an in-vitro ADCC model. Direct monocyte mediated cytotoxicity and ADCC activity was detected before and during the cytokine treatment, but there was no significant enhancement after GM-CSF or rIFN-γ1b. These findings are consistent with our previous report that the proportion of low affinity receptor CD16+ MO/MA, which mediate direct cytotoxicity as well as ADCC, are not increased following GM-CSF treatment [27]. However, the expansion of MO, which includes CD16+ ADCC effector cells, on day 9 of each cycle could suggest that day 9 of the carboplatin/GM-CSF regimen might be an appropriate time to include suitable human chimerized anti-tumor cell monoclonal antibodies, that are able to both target tumor cells in-vivo and provide selective binding to CD16+ effector cells. The current trial had significant hematologic toxicity which we attributed to the addition of rIFN-γ1b. Since rIFN-γ1b failed to add any overt immuno-hematologic advantage and increased hematologic toxicity, we would not recommend further study of rIFN-γ1b in combination with carboplatin in this patient setting.
In summary, the 56% response rate observed in our study is higher than reported for most single-agent carboplatin trials, and overlaps with some combination regimens. This regimen is also reasonably well tolerated when compared with other carboplatin-based therapies in women with recurrent platinum-sensitive ovarian cancer. While GM-CSF continues to undergo development as an immunomodulatory agent, the clinical-hematological profile of carboplatin plus GM-CSF could suggest a platform for possible future exploration in ovarian cancer patients. This might include GM-CSF combined sequentially with chemotherapy to increase monocyte and other effector cell levels, or the possibility of incorporating a target specific cell-surface antigen antibody that can mediate ADCC.
Acknowledgments
We thank Michael Garcia, RN, BSN for his recruitment of patients, collection of data and commitment to the study. In addition, we thank Maurie Markman, MD for his critical reading of the manuscript and many helpful suggestions.
Role of Funding Source: This study was supported in part by Bayer Healthcare Pharmaceuticals, Inc., Bristol-Myers Squibb Company, Inc. and InterMune, Inc. These companies had no role in designing the study; collecting, analyzing, or interpreting data; or writing this report.
Footnotes
Presented in part at the annual meeting of the Society of Gynecologic Oncologists, Tampa, Florida, March 2008.
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL., Jr Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 2.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Trope C. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Martin AJ, Calvo E, Bover I, Rubio MJ, Arcusa A, Casado A, Ojeda B, Balana C, Martinez E, Herrero A, Pardo B, Adrover E, Rifa J, Godes MJ, Moyano A, Cervantes A. Randomized phase II trial of carboplatin versus paclitaxel and carboplatin in platinum-sensitive recurrent advanced ovarian carcinoma: a GEICO (Grupo Espanol de Investigacion en Cancer de Ovario) study. Ann Oncol. 2005;16:749–55. doi: 10.1093/annonc/mdi147. [DOI] [PubMed] [Google Scholar]
- 4.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stahle A, Stuart G, Kimmig R, Olbricht S, Le T, Emerich J, Kuhn W, Bentley J, Jackisch C, Luck HJ, Rochon J, Zimmermann AH, Eisenhauer E. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 5.Strauss HG, Henze A, Teichmann A, Karbe I, Baumgart A, Thomssen C, Koelbl H. Phase II trial of docetaxel and carboplatin in recurrent platinum-sensitive ovarian, peritoneal and tubal cancer. Gynecol Oncol. 2007;104:612–6. doi: 10.1016/j.ygyno.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero JM, Weber B, Geay JF, Lepille D, Orfeuvre H, Combe M, Mayer F, Leduc B, Bourgeois H, Paraiso D, Pujade-Lauraine E. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: a GINECO phase II trial. Ann Oncol. 2007;18:263–8. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 7.Triozzi PL, Tucker F, Benzies T, Balcerzak SP. Antitumor and accessory immune activities of peripheral blood stem cells mobilized with granulocyte-macrophage colony-stimulating factor. Bone Marrow Transplant. 1996;18:47–52. [PubMed] [Google Scholar]
- 8.Allavena P, Peccatori F, Maggioni D, Erroi A, Sironi M, Colombo N, Lissoni A, Galazka A, Meiers W, Mangioni C, et al. Intraperitoneal recombinant gamma-interferon in patients with recurrent ascitic ovarian carcinoma: modulation of cytotoxicity and cytokine production in tumor-associated effectors and of major histocompatibility antigen expression on tumor cells. Cancer Res. 1990;50:7318–23. [PubMed] [Google Scholar]
- 9.Grabstein KH, Urdal DL, Tushinski RJ, Mochizuki DY, Price VL, Cantrell MA, Gillis S, Conlon PJ. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–8. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 10.Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, Soong SJ. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–21. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 11.Buter J, Pinedo HM. Neoadjuvant chemoimmunotherapy in locally advanced breast cancer: a new avenue to be explored. Curr Oncol Rep. 2003;5:171–6. doi: 10.1007/s11912-003-0106-7. [DOI] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Guastalla JP, Colombo N, Devillier P, Francois E, Fumoleau P, Monnier A, Nooy M, Mignot L, Bugat R, Marques C, Mousseau M, Netter G, Maloisel F, Larbaoui S, Brandely M. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol. 1996;14:343–50. doi: 10.1200/JCO.1996.14.2.343. [DOI] [PubMed] [Google Scholar]
- 13.Windbichler GH, Hausmaninger H, Stummvoll W, Graf AH, Kainz C, Lahodny J, Denison U, Muller-Holzner E, Marth C. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82:1138–44. doi: 10.1054/bjoc.1999.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marth C, Windbichler GH, Hausmaninger H, Petru E, Estermann K, Pelzer A, Mueller-Holzner E. Interferon-gamma in combination with carboplatin and paclitaxel as a safe and effective first-line treatment option for advanced ovarian cancer: results of a phase I/II study. Int J Gynecol Cancer. 2006;16:1522–8. doi: 10.1111/j.1525-1438.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Marth C, Alvarez RD, Johnson G, Bidzinski M, Kardatzke DR, Bradford WZ, Loutit J, Kirn DH, Clouser MC, Markman M. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression-free survival. Gynecol Oncol. 2008;109:174–81. doi: 10.1016/j.ygyno.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Vadhan-Raj S, Broxmeyer HE, Hittelman WN, Papadopoulos NE, Chawla SP, Fenoglio C, Cooper S, Buescher ES, Frenck RW, Jr, Holian A, et al. Abrogating chemotherapy-induced myelosuppression by recombinant granulocyte-macrophage colony-stimulating factor in patients with sarcoma: protection at the progenitor cell level. J Clin Oncol. 1992;10:1266–77. doi: 10.1200/JCO.1992.10.8.1266. [DOI] [PubMed] [Google Scholar]
- 17.Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–56. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Markman M, Markman J, Webster K, Zanotti K, Kulp B, Peterson G, Belinson J. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol. 2004;22:3120–5. doi: 10.1200/JCO.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 20.Bast RC, Thigpen JT, Arbuck SG, Basen-Engquist K, Burke LB, Freedman R, Horning SJ, Ozols R, Rustin GJ, Spriggs D, Wenzel LB, Pazdur R. Clinical trial endpoints in ovarian cancer: report of an FDA/ASCO/AACR Public Workshop. Gynecol Oncol. 2007;107:173–6. doi: 10.1016/j.ygyno.2007.08.092. [DOI] [PubMed] [Google Scholar]
- 21.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 22.Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Scales, Center on Outcomes, Research and Education (CORE) Evanston Northwestern Healthcare and Northwestern University, 1997; 1997. [Google Scholar]
- 23.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Murphy BA, Bacik J, Schwartz LH, Nanus DM, Mariani T, Loehrer P, Wilding G, Fairclough DL, Cella D, Mazumdar M. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18:2972–80. doi: 10.1200/JCO.2000.18.16.2972. [DOI] [PubMed] [Google Scholar]
- 25.Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. J Clin Oncol. 2003;21:366–73. doi: 10.1200/JCO.2003.02.136. [DOI] [PubMed] [Google Scholar]
- 26.Melichar B, Savary CA, Patenia R, Templin S, Melicharova K, Freedman RS. Phenotype and antitumor activity of ascitic fluid monocytes in patients with ovarian carcinoma. Int J Gynecol Cancer. 2003;13:435–43. doi: 10.1046/j.1525-1438.2003.13331.x. [DOI] [PubMed] [Google Scholar]
- 27.Apte SM, Vadhan-Raj S, Cohen L, Bassett RL, Gordon IO, Levenback CF, Ramirez PT, Gallardo ST, Patenia RS, Garcia ME, Iyer RB, Freedman RS. Cytokines, GM-CSF and IFNgamma administered by priming and post-chemotherapy cycling in recurrent ovarian cancer patients receiving carboplatin. J Transl Med. 2006;4:16. doi: 10.1186/1479-5876-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Johnson S, Bonvini E, Koenig S. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–90. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–81. [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Vermorken JB, van Glabbeke M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients [seecomments] Ann Oncol. 1997;8:963–8. doi: 10.1023/a:1008240421028. [DOI] [PubMed] [Google Scholar]
- 31.Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 32.Polyzos A, Tsavaris N, Kosmas C, Arnaouti T, Kalahanis N, Tsigris C, Giannopoulos A, Karatzas G, Giannikos L, Sfikakis PP. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129–33. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 33.Navo M, Kunthur A, Badell ML, Coffer LW, 2nd, Markman M, Brown J, Smith JA. Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol. 2006;103:608–13. doi: 10.1016/j.ygyno.2006.04.002. [DOI] [PubMed] [Google Scholar]


