Abstract
Background
Cataract formation often occurs in people with uveitis. It is unclear which intraocular lens (IOL) type is optimal for use in cataract surgery for eyes with uveitis.
Objectives
To summarize the effects of different IOLs on visual acuity, other visual outcomes, and quality of life in people with uveitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2013, Issue 7), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2013), EMBASE (January 1980 to August 2013), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to August 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 August 2013. We also performed forward and backward searching using the Science Citation Index and the reference lists of the included studies, respectively, in August 2013.
Selection criteria
We included randomized controlled trials (RCTs) comparing hydrophobic or hydrophilic acrylic, silicone, or poly(methyl methacrylate) (PMMA) IOLs with or without heparin-surface modification (HSM), with each other, or with no treatment in adults with uveitis, for any indication, undergoing cataract surgery.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration. Two review authors screened the search results and for included studies, assessed the risk of bias and extracted data independently. We contacted study investigators for additional information. We did not perform a meta-analysis due to variability in reporting and follow-up intervals for the primary and secondary outcomes of interest.
Main results
We included four RCTs involving 216 participants (range of 2 to 140 participants with uveitic cataract per trial) and comparing up to four types of IOLs. The largest study was an international study with centers in Brazil, Egypt, Finland, France, Japan, the Netherlands, Slovak Republic, Spain, and the USA; two studies were conducted in Germany and one in Saudi Arabia. There was substantial heterogeneity with respect to the ages of participants and etiologies of uveitis within and across studies. The length of follow-up among the studies ranged from 1 to 24 months after cataract surgery. The studies were at low risk of selection bias, but two of the four studies did not employ masking and only one study included all randomized participants in the final analyses. The funding source was disclosed by investigators of the largest study (professional society) and not reported by the other three. Due to heterogeneity in lens types evaluated and outcomes reported among the trials, we did not combine data in a meta-analysis.
In the largest study (140 participants), the study eye of each participant was randomized to receive one of four types of IOLs: hydrophobic acrylic, silicone, HSM PMMA, or unmodified PMMA. Proportions of participants with one or more Snellen lines of visual improvement were similar among the four treatment groups at one year' follow-up: 45 of 48 (94%) in the hydrophobic acrylic IOL group, 39 of 44 (89%) in the silicone IOL group, 18 of 22 (82%) in the HSM PMMA IOL group, and 22 of 26 (85%) in the unmodified PMMA IOL group. When comparing hydrophobic acrylic IOLs with silicone IOLs, the risk ratio (RR) was 1.06 (95% confidence interval (CI) 0.93 to 1.20). At one year' follow-up, fewer eyes randomized to hydrophobic acrylic IOLs developed posterior synechiae when compared with eyes receiving silicone IOLs (RR 0.18, 95% CI 0.04 to 0.79); the effects between these groups were less certain with respect to developing posterior capsule opacification (PCO) (RR 0.74, 95% CI 0.41 to 1.37), corneal edema (RR 0.49, 95% CI 0.22 to 1.12), cystoid macular edema (RR 0.10, 95% CI 0.01 to 1.84), or mild IOL decentration (RR 0.92, 95% CI 0.06 to 14.22).
Two intra-individual studies also compared HSM PMMA IOLs with unmodified PMMA IOLs at three or six months of follow-up. These studies, including a combined total of 16 participants with uveitis, were insufficiently powered to detect differences in outcomes among eyes of people with uveitis randomized to receive HSM PMMA IOLs when compared with fellow eyes receiving unmodified PMMA IOLs.
In the fourth study (60 participants), the study eye of each participant was randomized to receive a hydrophobic or hydrophilic acrylic IOL. At three months, there were no statistical or clinical differences between hydrophobic and hydrophilic acrylic IOL types in the proportions of participants with two or more Snellen lines of visual improvement (RR 1.03, 95% CI 0.87 to 1.22). There were similar rates in the development of PCO between hydrophobic or hydrophilic acrylic IOLs at six months' follow-up (RR 1.00, 95% CI 0.80 to 1.25). The effect of the lenses on posterior synechiae was uncertain at six months' follow-up (RR 0.50, 95% CI 0.05 to 5.22).
None of the included studies reported quality of life outcomes.
Authors' conclusions
Based on the trials identified in this review, there is uncertainty as to which type of IOL provides the best visual and clinical outcomes in people with uveitis undergoing cataract surgery. The studies were small, not all lens materials were compared in all studies, and not all lens materials were available in all study sites. Evidence of a superior effect of hydrophobic acrylic lenses over silicone lenses, specifically for posterior synechiae outcomes comes from a single study at a high risk of performance and detection bias. However, due to small sample sizes and heterogeneity in outcome reporting, we found insufficient information to assess these and other types of IOL materials for cataract surgery for eyes with uveitis.
Background
Description of the condition
Uveitis is a complex intraocular inflammatory disease of the middle part of the eye. A wide variety of causes exists for uveitis, including autoimmune processes and infectious agents (McCannel 1996). Nevertheless, the majority of uveitis cases are not associated with systemic diseases (Muñoz-Fernández 2006).
About 1% of the US population has uveitis (Gritz 2004). This condition appears to increase with age, with the highest incidence rates seen in older people (Gritz 2004; Reeves 2006).
Uveitis significantly impacts people's lives. It is thought to be responsible for approximately 5% to 20% of legal blindness in developed countries (Bodaghi 2001). Many people with uveitis may also experience blurred vision, sensitivity to light, eye pain, dark floating spots, and redness.
One of the common complications of uveitis is cataract formation (Okhravi 1999). While the incidence of cataract varies according to the type of uveitis, it is potentially as high as 50% (Rojas 1997). Unlike the general public, people with uveitis often develop cataract at an earlier stage in life. Prior intraocular inflammation and corticosteroid use are thought to be associated with cataract development in individuals with uveitis (Alió 1999).
Description of the intervention
Intraocular lens (IOL) insertion for cataracts is one of the most commonly performed surgical procedures (Woodcock 2004). An IOL is an implant placed in the eye at the time the cataractous crystalline lens is removed. During the early 1980s, the vast majority of practicing ophthalmologists considered it poor judgment to place an IOL in uveitic eyes (Lichter 1989). It was reasoned that there was not enough experience with IOLs to know whether uveitic eyes would tolerate them over many years. As increasing numbers of implants have been performed, considerable relaxation of the contraindications occurred (Lichter 1989).
Since the 1990s, a great deal of discussion has centered on the surgical correction of cataracts in people with uveitis including indications and timing of surgery, optimal surgical technique, and pre- and postoperative surgical treatment for cataract extraction (Alió 1999; Dunn 2009; Foster 1992; Okhravi 1999; Van Gelder 2009). In the early 2000s, phacoemulsification with in-the-bag IOL implantation emerged as a preferred surgical procedure for most people with uveitis with cataract (Alió 2002). The surgery is often performed under local anesthesia with the person in ‘twilight sleep’ (not fully awake or fully sedated).
How the intervention might work
Poly(methyl methacrylate) (PMMA), a rigid, transparent plastic as known as Plexiglas or Lucite, was the first material for IOLs (Kang 2008). Use of PMMA for IOLs has become superseded by flexible (or foldable) materials. The use of a more flexible IOL has allowed for a smaller incision, avoidance of stitches, shorter surgical time, and speedier postoperative recovery. It is believed that utilization of a flexible IOL placed in the intact capsular bag results in less surgical trauma and surgically induced inflammation than alternative approaches (Rojas 1996). Current flexible materials include silicone and acrylic (Bellucci 2013).
IOL materials are further distinguished as being either hydrophobic (repels water) or hydrophilic (combines or attracts water) based on the angle a water drop makes on the materials surface (hydrophobic materials result in greater contact angles and hydrophilic materials have more acute contact angles with water). Whether a lens is hydrophobic or hydrophilic is thought to affect the biocompatibility of the IOL in the eye, especially in people with history of ocular inflammation such as uveitis. Because the lens is surrounded by aqueous humor in the eye, it has been suggested that hydrophilic materials could be more biocompatible than hydrophobic materials in eyes with uveitis (Abela-Formanek 2002; Alió 2002). In addition, hydrophilic IOLs have been postulated to reduce electrostatic forces and cellular adhesion, consequently preventing the attraction of inflammatory cells and adherence of fibroblasts to the surface of the IOL (Kang 2008; Tabbara 1998). PMMA lOLs are inherently hydrophobic, but can be modified by adding heparin to the surface of the lens to become hydrophilic. Silicone IOLs are hydrophobic. Acrylic IOLs may be hydrophobic or hydrophilic depending on the compounds in the material. Hydrophilic acrylic materials also may be referred to as hydrogel IOLs.
Why it is important to do this review
The use of flexible IOLs has increased compared with the traditional PMMA lens, but it is still unclear which IOL material (PMMA, silicone, acrylic) is optimal for use in cataract surgery for eyes with uveitis, or whether approaches such as heparin-surface modification (HSM) are beneficial (Alió 2002). This systematic review evaluated the safety and efficacy of various IOLs for the treatment of cataract in people with uveitis.
Objectives
To summarize the effects of different IOLs on visual acuity, other visual outcomes, and quality of life in people with uveitis.
Methods
Criteria for considering studies for this review
Types of studies
We followed the methods set forth in the published protocol for this review (Ssemanda 2008). We included randomized controlled trials (RCTs) with at least three months of follow-up after cataract surgery.
Types of participants
We included trials that enrolled adults (18 years and older) with cataract developing after uveitis for any indication. We included studies that did not report the age of participants and studies that included some participants less than 18 years of age. We included studies in which only a subset of participants were eligible (i.e. had uveitic cataract) when outcome data were reported separately for this subset.
Types of interventions
We included trials comparing hydrophobic or hydrophilic acrylic, silicone, or PMMA IOLs with or without HSM with each other, or with treatment.
Types of outcome measures
Primary outcomes
We considered that treatment was administered to reverse visual impairment. Thus, the primary outcome was the proportion of participants whose best-corrected visual acuity (BCVA) improved at least five letters on the Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity chart, or the equivalent changes on the Snellen chart or other scales at least three months postoperatively by lens type.
Secondary outcomes
Secondary outcomes at least three months postoperatively included the:
proportion of participants requiring additional interventions by lens type;
proportion of participants with posterior capsule opacification (PCO) postoperatively by lens type;
proportion of participants with posterior synechiae by lens type;
proportion of participants with increased inflammation by lens type;
proportion of participants with an improvement in quality of life as measured by a validated scale by lens type;
proportion of participants with adverse events (e.g. corneal edema, cystoid macular edema (CME), mild IOL decentration) by lens type.
We also considered longer times of outcome assessment when available (i.e. six months, 12 months, etc.).
As a post-hoc decision, we decided to include additional visual acuity measures we felt represented patient-important outcomes:
proportion of participants with BCVA 20/40 or better(Snellen equivalent): in the US, BCVA of 20/40 is the minimum visual acuity requirement for possessing a unrestricted driver's license;
proportion of participants with BCVA 20/200 or worse (Snellen equivalent): the generally accepted definition of legal blindness in most countries.
Due to the paucity of visual acuity outcome data available, we also included mean change in BCVA as a secondary outcome. When mean change in BCVA was not reported, we used mean BCVA at a follow-up time point.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) 2013, Issue 7, part of The Cochrane Library. www.thecochranelibrary.com (accessed 14 August 2013), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2013), EMBASE (January 1980 to August 2013), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to August 2013), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 August 2013.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of included studies to identify any additional trials. We also used the Science Citation Index - Expanded database to identify additional trials that may have cited any studies that we included in the review.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts of all records identified through the electronic searches. Each review author assessed records for eligibility and classified each record as 1) include, 2) unclear, or 3) exclude. We resolved discrepancies by discussion. We obtained the full-text records for studies appearing to meet the inclusion criteria (1) or for which there were insufficient data in the title and abstract to make a clear decision (2). Two review authors independently assessed full-text records obtained from all the electronic and other methods of searching to establish whether the studies met the inclusion criteria. We resolved discrepancies by discussion. When resolution was not possible, we consulted a third review author. Studies excluded after full-text assessment appear in the Characteristics of excluded studies table with reasons for exclusion recorded. We attempted to contact trial investigators when data provided by the records were insufficient to include or exclude the study from the review. Studies for which we received no clarification from trial investigators are listed in the Characteristics of studies awaiting classification table.
Data extraction and management
Two review authors independently extracted primary and secondary outcome findings for included studies onto paper data collection forms developed and piloted in collaboration with the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. We contacted trial investigators of included studies for missing data. Six weeks was provided for primary study investigators to respond. One review author entered all data into Review Manager 5 (RevMan 2012). A second review author verified the data entered.
For each trial, we recorded the following:
year of publication, country of origin, and source of study funding;
details of the study methods relevant to assessing risks of bias;
details of the participants including demographic characteristics and criteria for inclusion;
details of the types of interventions;
details of the outcomes reported, including method of assessment, and time intervals.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for the included trials as part of the data extraction process. We followed the tools for assessing risk of bias set forth in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We examined six main criteria for each study:
adequate sequence generation;
adequate allocation concealment;
adequate masking of participants, personnel, and outcome assessors;
incomplete outcome data adequately addressed;
free of selective outcome reporting; and
free of other sources of bias.
We assessed each risk of bias criteria as:
low risk of bias (plausible bias unlikely to seriously alter the results);
unclear risk of bias (lack of information or uncertainty over the potential for bias); and
high risk of bias (plausible bias that seriously weakens confidence in the results).
We requested additional information from trial investigators when methods to access risks of bias were not reported. Further methodological assessments included sample size calculations, whether there was a clear explanation that final visual acuity took into account differential follow-up, mention of the exclusion/inclusion criteria, adequate definitions of success criteria, and comparability of control and treatment groups at entry.
Measures of treatment effect
When visual acuity was treated as a continuous outcome, we expressed treatment effect as a difference in means with 95% confidence intervals (CI). For dichotomous outcomes, we expressed the estimates of effect of an intervention as risk ratios (RR) with 95% CIs. Dichotomous outcomes included the proportion of participants whose BCVA improved at least five letters, the proportion of participants with BCVA 20/40 or better, the proportion of participants with BCVA 20/200 or worse (Snellen equivalent), the proportion of participants requiring additional interventions, the proportion of participants with PCO postoperatively, the proportion of participants with posterior synechiae, the proportion of participants with increased inflammation, the proportion of participants with an improvement in quality of life, and the proportion of participants with adverse events.
Unit of analysis issues
The unit of analysis was the individual for two studies in which only one eye of each participant was included in the study. For two studies with an intra-individual design, the unit of analysis was the eye for outcomes of visual acuity, PCO, additional postoperative interventions, and adverse events. Neither of these studies made appropriate adjustments for intra-person correlation between eyes as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We analyzed the data as available and noted the limitations with these interpretations.
With respect to studies with more than two intervention arms, we analyzed data by the groups to which participants were randomized. This method results in multiple, correlated, pair-wise comparisons for a given study. We have not adjusted for these correlations since no meta-analysis was done.
Dealing with missing data
At the review level, we attempted to contact trial investigators for any missing or unclear data. If investigators did not respond within six weeks, we extracted data as available from the published report. We referred to the guidelines for missing data in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
At the participant level, we reported the available-case data assuming data were missing at random. We did not perform any imputation of data.
Assessment of heterogeneity
We did not perform assessments of heterogeneity since we did not include meta-analyzed estimates of the treatment effect in this review. If additional studies are added during future updates to this review, we will quantify the proportion of variability across the included studies that is not due to chance by using the I2 statistic (Higgins 2011). If the I2 statistic is greater than 50%, we will consider there to be substantial statistical heterogeneity and will not combine the study results in a meta-analysis. Instead, we will present the studies in a tabulated, narrative summary. We will investigate heterogeneity, if present, through subgroup analyses when data are available.
Assessment of reporting biases
Since we did not include meta-analyzed estimates of treatment effect across studies, we did not examine funnel plots to identify evidence of publication bias.
Data synthesis
We did not perform meta-analysis due to variability in reporting and follow-up intervals for the primary and secondary outcomes of interest. If data synthesis is considered during a future update of this review, we will follow the guidelines for analysis set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We will calculate summary RRs for dichotomous outcomes when no substantial statistical heterogeneity is detected among included studies (an I2 statistic 50% or less). If there is a small number of included studies (fewer than three), we will use the fixed-effect model. We will use the random-effects model for analyses with three or more studies.
Subgroup analysis and investigation of heterogeneity
We found insufficient data to conduct subgroup analyses.
Sensitivity analysis
We did not conduct sensitivity analyses to determine the impact of excluding studies with lower methodological quality, including exclusion of industry-funded studies and unpublished studies since we included no studies in a meta-analysis.
Results
Description of studies
Results of the search
The electronic searches identified 893 titles and abstracts as of 14 August 2013, of which 33 records appeared to be relevant (Figure 1). We obtained the full-text copies of the 33 records, which reported 22 unique studies. We included four studies (Characteristics of included studies), excluded 16 studies, and identified two potentially relevant registered trials. Currently, no reports of the registered trials have been published and no replies from the trial investigators have been received (NCT00001311; NCT00000119). Based on available information, we have placed these two trials in the awaiting classification category and their descriptions are in the Characteristics of studies awaiting classification table.
Figure 1.
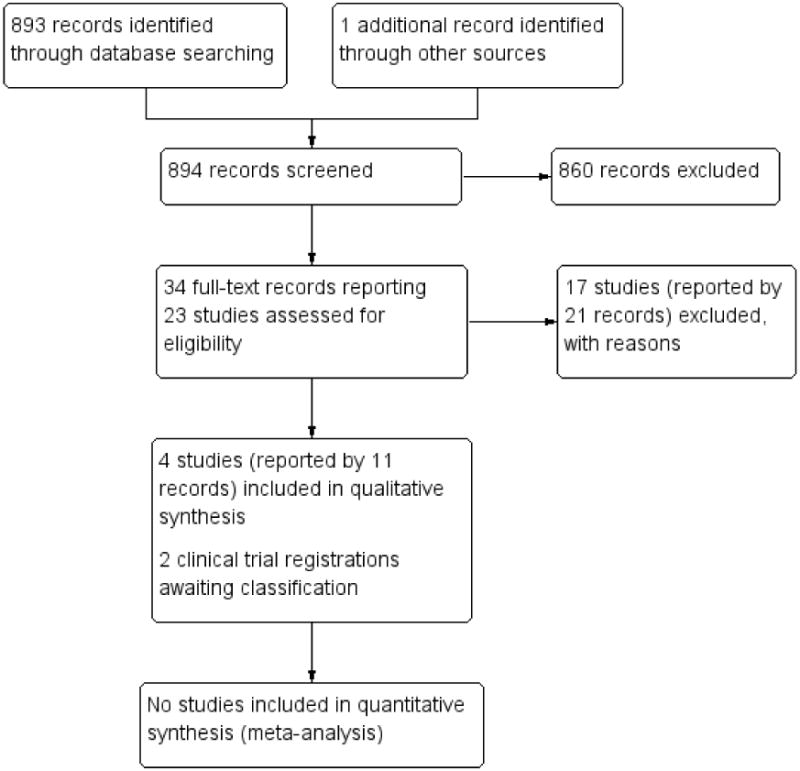
Study flow diagram.
Additionally, we identified one potentially relevant citation from a review of the reference lists of included studies, but after retrieving the abstract, we excluded this study. Thus, 17 excluded studies are listed in the Characteristics of excluded studies table with reasons for exclusions. Searches of the Science Citation Index - Expanded database as of 28 August 2013 did not yield any additional potentially relevant studies.
Included studies
Types of participants
The four studies included in this review enrolled 216 participants with uveitic cataract, with the largest enrolling 140 participants (Alió 2002). The Alió 2002 study was conducted at 19 international centers in Brazil, Egypt, Finland, France, Japan, the Netherlands, Slovak Republic, Spain, and the USA. Two studies, Mester 1998 and Roesel 2008, were conducted in Germany and the Tabbara 1998 study was done in Saudi Arabia. In Alió 2002, participants were aged 18 and older and had quiescent (inactive) uveitis for at least three months prior to cataract surgery. After one year of follow-up, outcomes for 64 men and 76 women were analyzed. Roesel 2008 enrolled 60 non-infectious uveitis participants (18 men and 42 women) with clinically significant lens opacities.
The two other included trials involved participants with multiple comorbidities undergoing cataract surgery. Mester 1998 included people with diabetes mellitus with or without retinopathy, glaucoma, pseudoexfoliation, and uveitis; ages of participants were not listed. There were two people with uveitis among the 100 participants enrolled in the study. Tabbara 1998 included 14 people with uveitis (three men, 11 women), aged 11 to 66 years, among 25 participants with diabetes mellitus or inactive uveitis.
Types of interventions
Alió 2002 randomized one eye of each participant to receive a hydrophobic acrylic (48 participants), silicone (44 participants), unmodified PMMA (26 participants), or HSM PMMA IOL (22 participants). Roesel 2008 randomized one eye to receive a hydrophobic acrylic or hydrophilic acrylic IOL. Mester 1998 and Tabbara 1998 randomized one eye to receive a HSM PMMA IOL and the fellow eye to receive an unmodified PMMA IOL.
Types of outcomes
The follow-up times for the four included studies ranged from less than one month to 24 months after surgery. Two studies, Alió 2002 and Roesel 2008, included visual acuity outcomes and reported the number of eyes requiring additional interventions, specifically Nd:YAG laser capsulotomy for PCO. Three studies, Alió 2002, Roesel 2008, and Tabbara 1998, reported the number of eyes developing PCO and the number of eyes developing posterior synechiae. All four included studies reported measures of postoperative inflammation. None of the included studies reported quality of life outcomes.
Alió 2002 reported outcomes for BCVA (mean BCVA, categories of BCVA, and number of Snellen lines gained or lost), postoperative inflammation, PCO, relapses, and other clinical observations (i.e. corneal edema, CME, pupillary membrane crossing, posterior synechiae, giant and small cells on the IOL optic, pigment deposits, and IOL decentration) at one year' follow-up. Roesel 2008 reported BCVA (mean BCVA and two or more lines of improvement), uveal biocompatibility (giant cells on IOL, anterior chamber cells, laser flare photometry, and posterior synechiae), and capsular biocompatibility (PCO, number of Nd:YAG laser capsulotomies, and lens epithelial cell outgrowth) at one, three, and six months after surgery. Adverse events including endophthalmitis, elevated intraocular pressure, and macular edema were also reported.
Tabbara 1998 reported outcomes for posterior synechiae, IOL cellular deposits, posterior capsular fibrosis, and anterior chamber reaction assessments six months after surgery. Mester 1998 reported the mean difference (MD) between preoperative flare and follow-up assessments at one day, one week, six weeks, and three months after surgery.
Excluded studies
We excluded 17 studies and the reasons for exclusion may be found in the Characteristics of excluded studies table. Thirteen studies were not RCTs, three studies excluded people with uveitis, and one study did not specify the types of IOLs used.
Risk of bias in included studies
Figure 2 shows the risk of bias assessments for all included studies. Details for each risk of bias parameter by study are provided in the Characteristics of included studies table.
Figure 2.
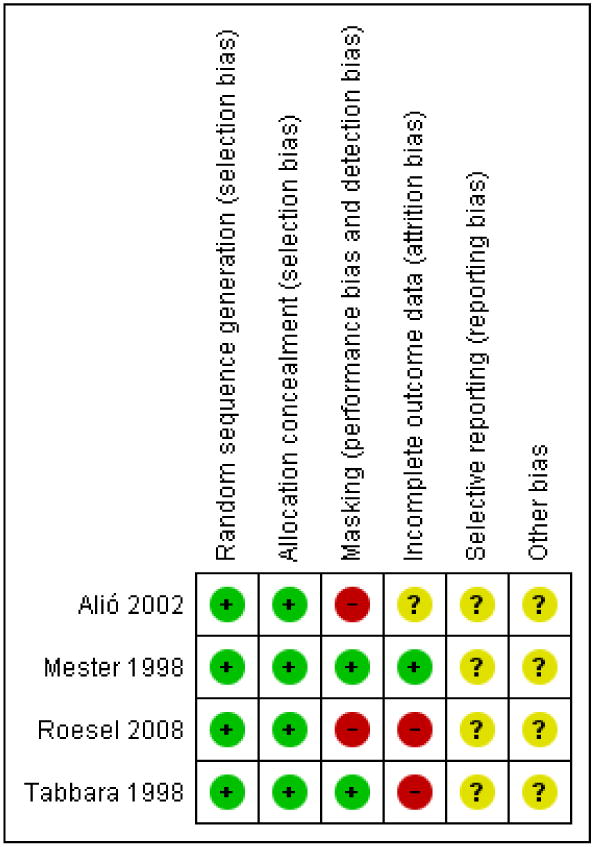
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Sequence generation
Alió 2002 employed a complete block design; data coordinating center staff were responsible for generating the random assignments. Mester 1998, Roesel 2008, and Tabbara 1998 used a computer to generate random assignments.
Allocation concealment
Alió 2002 used a complete block design with IOL assignment in sequentially numbered, sealed envelopes. In the Tabbara 1998 study, IOLs were concealed in boxes and opened during the procedure by an unmasked operating room nurse. Mester 1998 and Roesel 2008 reported using sealed envelopes.
Masking (performance bias and detection bias)
In Alió 2002, participants, surgeons, and outcome assessors were not masked to treatment assignment. In Tabbara 1998, participants and surgeons (same surgeons treated and followed participants) were masked to treatment assignment. The research coordinator and operating room nurse were unmasked. Mester 1998 reported that participants were masked. Roesel 2008 noted that surgeons and outcome assessors could not be masked due to obvious differences in IOL design.
Incomplete outcome data
In Alió 2002, of 170 people screened for the trial, 140 met the enrollment criteria and completed one year of follow-up. It is unknown whether there were additional enrolled participants who were not included in the report because they did not complete the one year' follow-up exam. In Tabbara 1998, three people with uveitis (6 eyes) missed the six-month follow-up visit and were not included in the final analyses (based on correspondence with the lead study author) and even after considering this exclusion, reports of PCO were reported for only 19 of the remaining 22 eyes. Roesel 2008 did not report losses or exclusions, but numbers and percentages of participants with visual acuity outcomes of interest suggested that data were missing for four eyes in the hydrophobic acrylic lens group and for one eye in the hydrophilic acrylic lens group at three months and five eyes in the hydrophobic acrylic lens group and six eyes in the hydrophilic acrylic lens group at six months. There were no apparent losses or exclusions in Mester 1998.
Selective reporting
There was insufficient information to determine whether any of the included studies were at high or low risk of bias due to selective outcome reporting.
Other potential sources of bias
There was insufficient information to determine whether there were other potential sources of bias.
Effects of interventions
The variability of interventions and outcomes (timing and measurement) among studies precluded meta-analysis. We report, instead, summaries of study results by comparisons of IOL types.
Acrylic versus silicone intraocular lenses
Visual acuity
One study, Alió 2002, compared hydrophobic acrylic IOLs (48 participants) with silicone IOLs (44 participants). The proportion of participants with one or more Snellen lines of visual improvement (equivalent of gaining five or more letters on the ETDRS visual acuity chart) in the study eye was similar between treatment groups at one year' follow-up: 45 of 48 (94%) eyes in the acrylic IOL group and 39 of 44 (89%) eyes in the silicone IOL group (RR 1.06, 95% CI 0.93 to 1.20) (Table 1).
Table 1. Visual acuity outcomes at one year by intraocular lens types in Alió 2002 study.
| Type of IOLs compared (number of participants) | BCVA, measured as Snellen equivalent | |||
|---|---|---|---|---|
| ≥ 1 lines gained RR (95% CI)* | ≥ 20/40 RR (95% CI)* | ≤ 20/200 RR (95% CI)** | Mean BCVA (Mean ± number of lines) | |
| Acrylic (48) versus silicone (44) | 1.06 (0.93 to 1.20) | 2.06 (1.20 to 3.55) | 0.57 (0.20 to 1.62) | (20/80 ± 2) versus (20/160 ± 1) (P value = 0. 006) |
| Acrylic (48) versus unmodified PMMA (26) | 1.11 (0.93 to 1.33) | 1.13 (0.71 to 1.78) | 0.68 (0.20 to 2.31) | (20/80 ± 2) versus (20/100 ± 2) (P value ≥ 0.05) |
| Acrylic (48) versus HSM PMMA (22) | 1.15 (0.93 to 1.41) | 1.13 (0.69 to 1.83) | 0.46 (0.15 to 1.42) | (20/80 ± 2) versus (20/120 ± 2) (P value ≥ 0.05) |
| Unmodified (26) PMMA versus silicone (44) | 0.95 (0.79 to 1.16) | 1.83 (0.99 to 3.40) | 0.85 (0.28 to 2.54) | (20/100 ± 2) versus (20/160 ± 1) (P value = 0.05) |
| HSM PMMA (22) versus silicone (44) | 0.92 (0.74 to 1.15) | 1.83 (0.97 to 3.47) | 1.25 (0.46 to 3.38) | (20/120 ± 2) versus (20/160 ± 1) (P value ≥ 0.05) |
| Unmodified (26) PMMA versus HSM PMMA (22) | 1.03 (0.80 to 1.34) | 1.00 (0.57 to 1.76) | 0.68 (0.21 to 2.22) | (20/100 ± 2) versus (20/120 ± 2) (P value ≥ 0.05) |
BCVA: best-corrected visual acuity; CI: confidence interval; HSM: heparin-surface modified; IOL: intraocular lens; PMMA: poly(methyl methacrylate); RR: risk ratio.
RRs > 1 favor the test IOL type and RRs < 1 favor the comparison IOL type.
RRs < 1 favor the test IOL type and RRs > 1 favor the comparison IOL type.
Mean BCVA at one year was significantly improved in eyes with acrylic IOLs (20/80 ± 2) when compared with silicone IOLs (20/ 160 ± 1) (P value = 0.006). We did not transform mean BCVA (± standard deviation) values to calculate a between-group difference because the Snellen scale is not linear. Participants in the acrylic IOL group were twice as likely to achieve BCVA of 20/40 or better in the study eye compared with participants in the silicone IOL group (RR 2.06, 95% CI 1.20 to 3.55). Five of 48 (10%) eyes in the acrylic IOL group and 8 of 44 (18%) eyes in the silicone IOL group had BCVA of 20/200 or worse (RR 0.57, 95% CI 0.20 to 1.62) (Table 1).
Additional interventions
Alió 2002 reported the number of eyes requiring additional interventions, specifically Nd:YAG laser capsulotomy for PCO. Up to one year of follow-up, a capsulotomy had been performed in 2 of 48 (4%) eyes randomized to acrylic IOLs (one each at three and six months) and 3 of 44 (7%) eyes randomized to silicone IOLs (two at three months and one at one year). Because of the small number of events in both groups, the 95% CI for this result was wide and the results may be unreliable (RR 0.61, 95% CI 0.11 to 3.49) (Table 2).
Table 2. Adverse outcomes at one year by intraocular lens types in Alió 2002 study.
| Type of IOLs compared (number of participants) | Additional interventions RR (95% CI)* | PCO RR (95% CI)* | Posterior synechiae RR (95% CI)* | Postoperative inflammation RR (95% CI)* | Corneal edemaRR (95% CI)* | CME RR (95% CI)* | Mild IOL decentration RR (95% CI)* |
|---|---|---|---|---|---|---|---|
| Acrylic (48) versus silicone (44) | 0.61 (0.11 to 3.49) | 0.74 (0.41 to 1.37) | 0.18 (0.04 to 0.79) | 0.72 (0.49 to 1.05) | 0.49 (0.22 to 1.12) | 0.10 (0.01 to 1.84) | 0.92 (0.06 to 14.22) |
| Acrylic (48) versus unmodified PMMA (26) | 2.76 (0.14 to 55.33) | 0.78 (0.39 to 1.58) | 2.76 (0.14 to 55.33) | 0.74 (0.48 to 1.15) | 3.79 (0.49 to 29.17) | 0.18 (0.01 to 4.36) | 1.65 (0.07 to 39.20) |
| Acrylic (48) versus HSM PMMA (22) | 2.35 (0.12 to 46.94) | 0.60 (0.31 to 1.14) | 0.46 (0.07 to 3.05) | 1.26 (0.67 to 2.37) | 1.07 (0.30 to 3.75) | 0 in both groups | 1.41 (0.06 to 33.26) |
| Unmodified (26) PMMA versus silicone (44) | 0.24 (0.01 to 4.43) | 0.95 (0.49 to 1.84) | 0.08 (0.00 to 1.30) | 0.97 (0.66 to 1.41) | 0.13 (0.02 to 0.94) | 0.42 (0.05 to 3.59) | 0.56 (0.02 to 13.16) |
| HSM PMMA (22) versus silicone (44) | 0.28 (0.02 to 5.18) | 1.25 (0.68 to 2.28) | 0.40 (0.10 to 1.67) | 0.57 (0.31 to 1.04) | 0.46 (0.15 to 1.45) | 0.22 (0.01 to 3.87) | 0.65 (0.03 to 15.39) |
| Unmodified (26) PMMA versus HSM PMMA(22) | 0 in both groups | 0.76 (0.38 to 1.53) | 0.17 (0.01 to 3.37) | 1.69 (0.90 to 3.18) | 0.28 (0.03 to 2.52) | 2.56 (0.11 to 59.75) | 0 in both groups |
CI: confidence interval; CME: cystoid macular edema; HSM: heparin-surface modified; IOL: intraocular lens; PCO: posterior capsule opacification; PMMA: poly(methyl methacrylate); RR: risk ratio.
RRs < 1 favor the test IOL type and RRs > 1 favor the comparison IOL type.
Posterior capsule opacification
At six months' follow-up, Alió 2002 reported that there were statistically fewer eyes developing PCO in the acrylic IOL group than the silicone IOL group (P value = 0.047; proportions not reported); however, by one year' follow-up, there was no statistically significant difference observed between groups as 13 of 48 (27%) eyes randomized to acrylic IOLs and 16 of 44 (36%) eyes randomized to silicone IOLs developed PCO of any grade (RR 0.74, 95% CI 0.41 to 1.37) (Table 2).
Posterior synechiae
Alió 2002 reported the number of eyes developing posterior synechiae. At one year' follow-up, fewer eyes developed posterior synechiae of any severity among those with acrylic IOLs (2 of 48, 4%) when compared with those with silicone IOLs (10 of 44, 23%) (RR 0.18, 95% CI 0.04 to 0.79) (Table 2).
Postoperative inflammation
Alió 2002 defined “an increase in the inflammatory recording of 2 degrees in Uveitic Scoring System scoring at two separate visits or at an unscheduled visit” as a relapse. Twenty-two of 48 (46%) eyes randomized to acrylic IOLs and 28 of 44 (64%) eyes with silicone IOLs experienced one or two relapses during the course of the one-year study (RR 0.72, 95% CI 0.49 to 1.05). A related measurement of postoperative inflammation was their grading of anterior chamber cell (inflammation) on postoperative days 1, 4, 7, and 15. The acrylic group had significantly less postoperative inflammation than the silicone group at days 1, 4, 7, and 15 (P value < 0.03 at all time points; proportions not reported) (Table 2).
Adverse events
Corneal edema developed in 7 of 48 (15%) eyes randomized to acrylic IOLs and 13 of 44 (30%) eyes with silicone IOLs (RR 0.49, 95% CI 0.22 to 1.12). None of 48 (0%) eyes in the acrylic IOL group compared with 4 of 44 (9%) eyes in the silicone IOL group developed CME (RR 0.10, 95% CI 0.01 to 1.84). One of 48 (0%) eyes with an acrylic IOL and 1 of 44 (2%) eyes with a silicone IOL experienced mild IOL decentration (RR 0.92, 95% CI 0.06 to 14.22) (Table 2).
Acrylic versus poly(methyl methacrylate) intraocular lenses
Visual acuity
One study, Alió 2002, compared hydrophobic acrylic IOLs (48 participants) with unmodified hydrophobic PMMA IOLs (26 participants) and hydrophilic heparin surface modified (HSM) PMMA IOLs (22 participants). At one year' follow-up, the proportion of participants with one or more Snellen lines of visual improvement (equivalent of gaining five or more letters on the ET-DRS visual acuity chart) in the study eye was similar in acrylic IOL group (45 of 48, 94%) compared with the unmodified PMMA IOL group (22 of 26, 85%) (RR 1.11, 95% CI 0.93 to 1.33) and the HSM PMMA IOL group (18 of 22, 82%) (RR 1.15, 95% CI 0.93 to 1.41) (Table 1).
Mean BCVA atone year was not significantly different among eyes with acrylic IOLs (20/80 ± 2) when compared with unmodified PMMA IOLs (20/100 ± 2) or HSM PMMA IOLs (20/120 ± 2). The proportion of participants with BCVA of 20/40 or better in the study eye was similar between the acrylic IOL group and unmodified PMMA IOL group (RR 1.13, 95% CI 0.71 to 1.78) and between the acrylic IOL group and HSM PMMA IOL group (RR 1.13, 95% CI 0.69 to 1.83). Five of 48 (10%) eyes in the acrylic IOL group versus 4 of 26 (15%) eyes in the unmodified PMMA IOL group (RR 0.68, 95% CI 0.20 to 2.31) and 5 of 22 (23%) eyes in the HSM PMMA IOL group (RR 0.46, 95% CI 0.15 to 1.42) had BCVA of 20/200 or worse (Table 1).
Additional interventions
Alió 2002 reported the number of eyes requiring Nd:YAG laser capsulotomy for PCO. Up to one year of follow-up, a capsulotomy had been performed in 2 of 48 (4%) eyes randomized to acrylic IOLs (one each at three and six months) versus 0 of 26 (0%) eyes in the unmodified PMMA IOL (RR 2.76, 95% CI 0.14 to 55.33) or 0 of 22 (0%) eyes in the HSM PMMA IOL (RR 2.35, 95% CI 0.12 to 46.94) groups (Table 2).
Posterior capsule opacification
At one year' follow-up, 13 of 48 eyes (27%) randomized to acrylic IOLs versus 9 of 26 (35%) randomized to unmodified PMMA IOLs (RR 0.78, 95% CI 0.39 to 1.58), and 10 of 22 (45%) randomized to HSM PMMA IOLs (RR 0.60, 95% CI 0.31 to 1.14) developed PCO of any grade (Table 2).
Posterior synechiae
At one year' follow-up, 2 of 48 (4%) eyes randomized to acrylic IOLs versus 0 of 26 (0%) eyes randomized to unmodified PMMA IOLs (RR 2.76, 95% CI 0.14 to 55.33) and 2 of 22 (9%) randomized to HSM PMMA IOLs (RR 0.46, 95% CI 0.07 to 3.05) developed posterior synechiae of any severity (Table 2).
Postoperative inflammation
During the course of one year, 22 of 48 (46%) eyes randomized to acrylic IOLs versus 16 of 26 (62%) eyes with unmodified PMMA IOLs (RR 0.74, 95% CI 0.48 to 1.15) and 8 of 22 (36%) eyes with HSM PMMA IOLs (RR 1.26, 95% CI 0.67 to 2.37) experienced one or two relapses, defined as “an increase in the inflammatory recording of 2 degrees in Uveitic Scoring System scoring at two separate visits or at an unscheduled visit”. One day postoperatively, anterior chamber cell grades were significantly lower in the acrylic IOL group than in the HSM PMMA group (P value = 0.026; proportions not reported); however, at all subsequent time points the trial authors reported there were no differences between groups (no quantitative data reported). The trial authors reported no significant differences in postoperative inflammation between the acrylic IOL group and the unmodified PMMA group at any period (Table 2).
Adverse events
Corneal edema developed in 7 of 48 (15%) eyes randomized to acrylic IOLs versus 1 of 26 (4%) eyes randomized to unmodified PMMA IOLs (RR 3.79, 95% CI 0.49 to 29.17) and 3 of 22 (14%) eyes randomized to HSM PMMA IOLs (RR 1.07, 95% CI 0.30 to 3.75). None of 48 (0%) eyes in the acrylic IOL group compared with 1 of 26 (4%) eyes in the unmodified PMMA IOL group (RR 0.18, 95% CI 0.01 to 4.36) developed CME. None of 22 (0%) eyes in the HSM PMMA IOL group developed CME. One of 48 (0%) eyes with an acrylic IOL, 0 of 26 (0%) eyes in the unmodified PMMA IOL, and 0 of 22 (0%) eyes in the HSM PMMA IOL group experienced mild IOL decentration (Table 2).
Poly(methyl methacrylate) versus silicone intraocular lenses
Visual acuity
One study, Alió 2002, compared unmodified hydrophobic PMMA IOLs (26 participants) or hydrophilic HSM PMMA IOLs (22 participants) with silicone IOLs (44 participants) (Table 1). At one year' follow-up, the proportion of participants with one or more Snellen lines of visual improvement (equivalent of gaining five or more letters on the ETDRS visual acuity chart) in the study eye was similar when comparing the unmodified PMMA IOL group (22 of 26, 85%) with the silicone IOL group (39 of 44, 89%) (RR 0.95, 95% CI 0.79 to 1.16) and the HSM PMMA IOL group (18 of 22, 82%) with the silicone IOL group (RR 0.92, 95% CI 0.74 to 1.15) (Table 1).
Mean BCVA at one year was reported to be significantly better among eyes with unmodified PMMA IOLs (20/100 ± 2) when compared with eyes with silicone IOLs (20/160 ± 1) (P value = 0.05). No significant difference was reported between eyes with HSM PMMA IOLs (20/120 ± 2) and eyes with silicone IOLs. The improvement in BCVA in eyes with unmodified PMMA IOLs (RR 1.83, 95% CI 0.99 to 3.40) or HSM PMMA IOLs (RR 1.83, 95% CI 0.97 to 3.47) was compatible with no difference and a substantial increase in BCVA of 20/40 or greater at one year when compared with eyes with silicone IOLs. Four of 26 (15%) eyes in the unmodified PMMA IOL group and 8 of 44 (18%) eyes in the silicone group had BCVA of 20/200 or worse (RR 0.85, 95% CI 0.28 to 2.54). Five of 22 (23%) eyes in the HSM PMMA IOL group compared with 4 of 44 (9%) in the silicone group had BCVA of 20/200 or worse (RR 1.25, 95% CI 0.46 to 3.38) (Table 1).
Additional interventions
Up to one year of follow-up, a capsulotomy had been performed in 0 of 26 (0%) eyes in the unmodified PMMA IOL (RR 0.24, 95% CI 0.01 to 4.43) and 0 or 22 (0%) eyes in the HSM PMMA IOL (RR 0.28, 95% CI 0.02 to 5.18) groups compared with 3 of 44 (7%) eyes randomized to silicone IOLs, two at three months and one at one year (Table 2).
Posterior capsule opacification
Alió 2002 reported that at six months there were a statistically fewer eyes developing PCO in the HSM PMMA IOL group than the silicone IOL group (P value = 0.05; proportions not reported).
At one year' follow-up, 9 of 26 (35%) eyes randomized to unmodified PMMA IOLs (RR 0.95, 95% CI 0.49 to 1.84) and 10 of 22 (45%) eyes randomized to HSM PMMA IOLs (RR 1.25, 95% CI 0.68 to 2.28) developed PCO of any grade compared with 16 of 44 (36%) eyes randomized to silicone IOLs (Table 2).
Posterior synechiae
At one year' follow-up, 0 of 26 (0%) eyes randomized to unmodified PMMA IOLs (RR 0.08, 95% CI 0.00 to 1.30) and 2 of 22 (9%) eyes randomized to HSM PMMA IOLs (RR 0.40, 95% CI 0.10 to 1.67) developed posterior synechiae of any severity compared with 10 of 44 (23%) eyes in the silicone IOL group (Table 2).
Postoperative inflammation
During the course of one year, 16 of 26 (62%) eyes with unmodified PMMA IOLs (RR 0.97, 95% CI 0.66 to 1.41) and 8 of 22 (36%) eyes with HSM PMMA IOLs (RR 0.57, 95% CI 0.31 to 1.04) experienced one or two relapses compared with 28 of 44 (64%) eyes with silicone IOLs. At one day, inflammation was reported to be significantly less in the unmodified PMMA group than in the silicone group (P value = 0.046; proportions not reported). There were no other significant differences among groups at any time (Table 2).
Adverse events
Corneal edema developed in 1 of 26 (4%) eyes randomized to unmodified PMMA IOLs (RR 0.13, 95% CI 0.02 to 0.94) and 3 of 22 (14%) eyes randomized to HSM PMMA IOLs (RR 0.46, 95% CI 0.15 to 1.45) compared with 13 of 44 (30%) eyes randomized to silicone IOLs. One of 26 (4%) eyes in the unmodified PMMA IOL group (RR 0.42, 95% CI 0.05 to 3.59) and 0 of 22 (0%) eyes in the HSM PMMA IOL group (RR 0.22, 95% 0.01 to 3.87) developed CME compared with 4 of 44 (9%) eyes in the silicone group. None of 26 (0%) eyes in the unmodified PMMA IOL group and 0 of 22 (0%) eyes in the HSM PMMA group experienced mild IOL decentration compared with 1 of 44 (2%) eyes in the silicone group (Table 2).
Unmodified versus heparin surface modified poly(methyl methacrylate) intraocular lenses
Visual acuity
Three studies, Alió 2002, Mester 1998, and Tabbara 1998, compared unmodified hydrophobic PMMA IOLs with hydrophilic HSM PMMA IOLs. In Alió 2002, 26 participants were randomized to unmodified PMMA IOLs and 22 participants were randomized to HSM PMMA IOLs with one study eye per participant (Table 1). Mester 1998 and Tabbara 1998 were paired-eye studies in which one eye of each participant was randomized to receive an unmodified PMMA IOL and the fellow eye received an HSM PMMA IOL. Both paired-eye studies included participants undergoing cataract surgery due to various etiologies, with four eyes of two participants from Mester 1998 and 28 eyes of 14 participants from Tabbara 1998 diagnosed with uveitis.
At one year follow-up in Alió 2002, the proportion of participants with one or more Snellen lines of visual improvement (equivalent of gaining five or more letters on the ETDRS visual acuity chart) in the study eye was similar between the unmodified PMMA IOL group (22 of 26, 85%) and the HSM PMMA IOL group (18 of 22, 82%) (RR 1.03, 95% CI 0.80 to 1.34). Mean BCVA at one year was not significantly different among eyes with unmodified PMMA IOLs (20/100 ± 2) or HSM PMMA IOLs (20/120 ± 2). The proportion of participants with BCVA of 20/40 or better in the study eye was similar between the unmodified PMMA IOL group and the HSM PMMA IOL group (RR 1.00, 95% CI 0.57 to 1.76). Four of 26 (15%) eyes in the unmodified PMMA IOL group and five of 22 (23%) eyes in the HSM PMMA IOL group had BCVA of 20/200 or worse (RR 0.68, 95% CI 0.21 to 2.22) (Table 1).
Mester 1998 and Tabbara 1998 did not report visual acuity outcomes.
Additional interventions
Alió 2002 reported no capsulotomies for PCO had been performed in eyes in either the unmodified PMMA IOL or HSM PMMA IOL groups at one year' follow-up (Table 2). Mester 1998 and Tabbara 1998 did not report outcomes for additional interventions.
Posterior capsule opacification
At six months' follow-up in Tabbara 1998, 5 of 9 (56%) eyes randomized to unmodified PMMA IOLs and 6 of 10 (60%) eyes randomized to HSM PMMA IOLs developed PCO (RR 0.93, 95% CI 0.43 to 2.01; no adjustments made for the non-independence of eyes).
At one year follow-up in Alió 2002, 9 of 26 (35%) eyes randomized to unmodified PMMA IOLs versus 10 of 22 (45%) eyes randomized to HSM PMMA IOLs developed PCO of any grade (RR 0.76, 95% CI 0.38 to 1.53) (Table 2). Mester 1998 did not report the number of eyes developing PCO.
Posterior synechiae
At six months' follow-up in Tabbara 1998, 2 of 11 (18%) eyes in the unmodified PMMA IOL group and 1 of 11 (9%) eyes in the HSM PMMA IOL group developed posterior synechiae (RR 2.00, 95% CI 0.21 to 18.98).
At one year' follow-up in Alió 2002, 0 of 26 (0%) eyes randomized to unmodified PMMA IOLs compared with 2 of 22 (9%) eyes randomized to HSM PMMA IOLs developed posterior synechiae of any severity (RR 0.17, 95% CI 0.01 to 3.37) (Table 2).
Mester 1998 did not report the number of eyes developing posterior synechiae.
Postoperative inflammation
At three months in the Mester 1998 study, the mean (standard deviation) difference in preoperative and three-month postoperative flare of two eyes with unmodified PMMA IOLs was 4.6 ± 0.6 photon counts/millisecond; the difference between the two eyes with HSM PMMA IOLs was -0.3 ± 10.8 (MD 4.90, 95% CI -10.09 to 19.89).
At six months in the Tabbara 1998 study, 7 of 11 (64%) eyes randomized to unmodified PMMA IOLs versus 4 of 11(36%) eyes randomized to HSM PMMA IOLs developed more than three cells on the anterior surface of the IOL. This difference was not statistically significant (RR 1.75, 95% CI 0.71 to 4.31).
During the course of one year in the Alió 2002 study, 16 of 26 (62%) eyes with unmodified PMMA IOLs compared with 8 of 22 (36%) eyes with HSM PMMA IOLs experienced one or two relapses, defined as “an increase in the inflammatory recording of 2 degrees in Uveitic Scoring System scoring at two separate visits or at an unscheduled visit” (RR 1.69, 95% CI 0.90 to 3.18). The trial authors reported no significant differences in postoperative inflammation between the unmodified PMMA IOL group and HSM PMMA IOL group at any period (Table 2).
Adverse events
Additional adverse events were reported in Alió 2002. Corneal edema developed in 1 of 26 (4%) eyes randomized to unmodified PMMAIOLsand3of22(14%)eyes randomized to HSM PMMA IOLs (RR 0.28, 95% CI 0.03 to 2.52). One of 26 (4%) eyes in the unmodified PMMA IOL group compared with 0 of 22 (0%) eyes in the HSM PMMA IOL group developed CME (RR 2.56, 95% CI 0.11 to 59.75). No eyes in either group experienced mild IOL decentration (Table 2).
Mester 1998 and Tabbara 1998 did not report additional complications.
Hydrophobic versus hydrophilic acrylic intraocular lenses
Visual acuity
One study, Roesel 2008, compared hydrophobic acrylic IOLs (30 participants) with hydrophilic acrylic IOLs (30 participants). At three months' follow-up visual acuity data were available for 26 of 30 (87%) participants in the hydrophobic acrylic IOL group and 29 of 30 (97%) participants in the hydrophilic acrylic IOL group. At six months' follow-up visual acuity data were available for 25 of 30 (83%) participants in the hydrophobic acrylic IOL group and 24 of 30 (80%) participants in the hydrophilic acrylic IOL group.
At three months, 24 of 26 (92%) eyes in the hydrophobic acrylic IOL group versus 26 of 29 (90%) eyes randomized to hydrophilic acrylic lenses had improvements in BCVA of two or greater Snellen lines (RR 1.03, 95% CI 0.87 to 1.22), suggesting no statistical or clinical difference between hydrophobic and hydrophilic acrylic IOL types. This outcome was maintained at six months: 21 of 25 (84%) eyes randomized to hydrophobic acrylic lenses versus 22 of 24 (92%) eyes randomized to hydrophilic acrylic lenses had improvements in BCVA of two or greater Snellen lines (RR 0.92, 95% CI 0.74, 1.13).
Mean BCVA did not significantly differ between group at three (MD 0.13 LogMAR, 95% CI -0.03 to 0.29) or six months (MD 0.10 LogMAR, 95% CI-0.08 to0.28). Roesel 2008 did not report outcomes for BCVA 20/40or better or for BCVA 20/200or worse.
Additional interventions
Roesel 2008 reported the number of eyes requiring additional interventions, specifically Nd:YAG laser capsulotomy for PCO. At six months, a capsulotomy had been performed in 6 of 30 (20%) eyes randomized to hydrophobic acrylic IOLs and 4 of 30 (13%) eyes randomized to hydrophilic IOLs (RR 1.50, 95% CI 0.47 to 4.78).
Posterior capsule opacification
At three months' follow-up, 17 of 30 (57%) eyes randomized to hydrophobic acrylic IOLs developed PCO compared with 22 of 30 (73%) eyes randomized to hydrophilic IOLs at three months (RR 0.77, 95% CI 0.53 to 1.13). An equal number of eyes developed PCO in both groups at six months (25 of 30 (83%) eyes in each group) (RR 1.00, 95% CI 0.80 to 1.25) (Roesel 2008).
Posterior synechiae
Over the course of the Roesel 2008 study, 1 of 30 (3%) eyes randomized to the hydrophobic IOL group and 2 of 30 (6%) eyes randomized to the hydrophilic IOL group developed posterior synechiae (RR 0.50, 95% CI 0.05 to 5.22).
Postoperative inflammation
Roesel 2008 reported the development of laser flare, anterior chamber cells, and giant cells on the anterior surface of the IOL. There were no differences reported in laser flare and anterior chamber cells at three and six months. Although the difference in giant cell deposits at three months was not significant (data not reported), at six months, eyes with hydrophobic acrylic IOLs (13 of 30 eyes, 42%) were 6.5 times more likely than eyes with hydrophilic acrylic IOLs (2 of 30 eyes, 8%) to have had giant cells graded as 1+ or more (RR 6.50, 95% 1.60 to 26.36). Three of 30 (10%) eyes with hydrophobic acrylic IOLs and 4 of 30 (13%) eyes with hydrophilic acrylic IOLs experienced reactivation of inflammation during the study (RR 0.75, 95% CI 0.18 to 3.07).
Adverse events
In Roesel 2008, 8 of 30 (27%) eyes with hydrophobic acrylic IOLs and 7 of 30 (23%) eyes with hydrophilic acrylic IOLs developed macular edema (RR 1.14, 95% CI 0.47 to 2.75); and 2 of 30 (7%) eyes with hydrophobic acrylic IOLs and 3 of 30 (10%) eyes with hydrophilic acrylic IOLs developed intraocular pressures of 22 mmHg or greater (RR 0.67, 95% CI 0.12 to 3.71). No eyes experienced IOL decentration, IOL dislocation, endophthalmitis, retinal detachment, or IOL exchange.
Discussion
Summary of main results
We identified four studies meeting the eligibility criteria for this review. The largest study suggested the use of hydrophobic acrylic IOLs over silicone IOLs in people over the age of 18 years with a history of uveitis in terms of improved BCVA (Alió 2002) (Table 1). Atone year, eyes randomized to hydrophobic acrylic IOLs were 2.06 times more likely (95% CI 1.20 to 3.55) to have had BCVA of 20/40 or greater when compared with eyes randomized to silicone IOLs; however, when visual acuity was measured as the proportion of participants with one or more Snellen lines of visual improvement, a difference between hydrophobic acrylic and silicone IOLs was not observed (RR 1.06, 95% CI 0.93 to 1.20). Fewer eyes with hydrophobic acrylic IOLs developed posterior synechiae of any severity or experienced postoperative inflammation compared with eye with silicone IOLs (Table 2). Eyes randomized to hydrophobic acrylic IOLs were reported to have been less likely to develop PCO six months postsurgery compared with eyes randomized to silicone IOLs (data not reported); however, this difference was less certain after one year (RR 0.74, 95% CI 0.41 to 1.37). Eyes randomized to hydrophobic acrylic IOLs also showed less postoperative inflammation compared with eyes randomized to silicone IOLs at 1, 4, 7, and 15 days after surgery. These two lens types were similar with respect to the proportions of participants requiring capsulotomy or experiencing other adverse events. In addition to acrylic and silicone IOLs, the Alió 2002 study investigated unmodified PMMA IOLs and HSM PMMA IOLs. Two other studies, Mester 1998 and Tabbara 1998, also compared unmodified PMMA IOLs and HSM PMMA IOLs. There were no statistically significant differences in outcomes among eyes of people randomized to receive HSM IOLs when compared with fellow eyes receiving PMMA IOLs; however, the small numbers of participants receiving these lens types may preclude the power to detect statistical differences between groups.
One study, Roesel 2008, compared hydrophobic acrylic IOLs with hydrophilic acrylic IOLs. At six months, the effects of the lens types in terms of BCVA, PCO, posterior synechiae, laser flare, or anterior chamber cells were uncertain due to a small sample size and wide CIs; however, 13 (42%) eyes randomized to hydrophobic versus two (8%) eyes randomized to hydrophilic acrylic IOLs developed 1+ or greater giant cell deposits on IOL (RR 6.50, 95% 1.60 to 26.36).
Quality of life outcomes were not reported in any study.
Overall completeness and applicability of evidence
Overall, the four studies specified different outcome measures, evaluated different IOLs, and were not consistent in elaborating the types or etiologies of uveitis. For example, the etiologies of uveitis in participants from the largest study were not reported (Alió 2002). Roesel 2008 specified that participants had “noninfectious” uveitis and listed the etiologies, such as “juvenile idiopathic arthritis, adult”. Juvenile idiopathic arthritis may overlap with juvenile rheumatoid arthritis, one of the two types of uveitis that was excluded from Alió 2002. Tabbara 1998 specified Behçet disease, Vogt-Koyanagi-Harada, and “non-granulomatous” uveitis. Although Alió 2002 argued that “ accurate patient selection fulfilling the inclusion and exclusion criteria, along with the preoperative and postoperative control of ocular inflammation and a standardized surgical procedure, nullified etiology as a variable that might affect the clinical results” and they “accordingly…found no need to group cases according to etiology”, etiology would be important to include as uveitis has ethnic/geographic predilections. Even without the inclusion of two types of uveitis in Alió 2002, there exists a wide range of types of uveitic disease, whose natural course could alter the results of cataract extraction with IOL. One study did not exclude children (Tabbara 1998), and the risks of cataract surgery with IOL implantation for children are different from adults undergoing surgery, including increased inflammatory response, secondary glaucoma, secondary membrane formation, and inadequate IOL power choice in a growing eye (Dharmaraj 2005; Hiles 1979; Trivedi 2006).
The rate of CME, an important outcome measure for cataract surgery, was mentioned in two studies (Alió 2002; Roesel 2008). Development of PCO in addition to the rate of Nd:YAG capsulotomy are other important outcomes that may reflect what Roesel termed, “capsular biocompatibility”. However, PCO development may also be a result of IOL design (sharp-edged optic versus other) and not reflective of material biocompatibility. “Relapse” (Alió 2002) or “reactivation” (Roesel 2008) of inflammation is another outcome measure for which the investigators of both studies used laser flare photometry, which was not included in the Mester 1998 or Tabbara 1998 studies. There was no mention of recurrence or relapse in Mester 1998 and Tabbara 1998, though the investigators may have chosen to use posterior synechiae and IOL cellular deposits as surrogate outcomes for inflammation. Alió 2002 and Roesel 2008 reported development of CME and relapse/reactivation of inflammation, and measured “giant cell” deposition on the IOL surface, which is not a standard outcome measure.
The publication dates of the four trials included in this review ranged from 1998 through 2008. Changes in materials and technology have evolved beyond the use of certain types of IOLs since then. For example, PMMA IOLs, which are rigid IOLs, have become superseded by flexible materials, such as acrylic, silicone, and hydrogel (Jancevski 2010).
Quality of the evidence
The studies included in this review were heterogeneous with respect to the types of IOLs evaluated (e.g. material of lenses, modification to surface of lenses), the underlying uveitic condition among study participants, the study methods and risks of bias, and the outcomes measured and reported. The number of participants included in the studies ranged from 2 to 140 (total of 216 participants); none of the studies reported sample size calculations needed to detect differences between treatment groups. The largest study in this review was a multicenter study in which not all study centers had access to all the types of lens implants, leading to varying numbers of participants in the four treatment groups (Alió 2002). The number of participants in the two types of PMMA groups was smaller compared with the hydrophobic acrylic and silicone IOL groups; it is unclear whether the study was powered to detect differences among these lenses. Further, outcome assessors in all four studies were not masked (although two studies measured only objective outcomes), uveitis type was not consistently provided, and outcome measures, types of lens material, follow-up periods, and patient populations were different across the studies, risking external validity for any one or all four studies.
Potential biases in the review process
In order to minimize potential selection bias in the review process, we devised a highly sensitive search strategy and reviewed records in duplicate by at least one clinician and one methodologist. Further, we abstracted data in duplicate and contacted study investigators when data were missing. However, we did not receive replies for all queries and some data are reported based on information as available. Due to the heterogeneity among studies and the lack of study protocols available, we could not assess potential selective outcome reporting or publication bias.
Although we feel that the systematic search for trials was comprehensive and selection bias was minimized, we identified only four trials with 216 total participants. This small number of study participants presents limitations to the interpretation of treatment effects including insufficient power to detect differences between treatment groups and unreliability in identifying adverse events.
Agreements and disagreements with other studies or reviews
There is a limited number of reviews regarding the optimal IOL to use in uveitic cataract surgery. However, the consensus of recent studies regarding cataract surgery in people with uveitis is that implantation of IOLs may be safely performed when ocular inflammation is well controlled. The Perry 2010 review concluded that acrylic and HSM PMMA lenses perform better in uveitic eyes than other lens types. This concurs with the conclusion from the Alió 2002 trial that an acrylic IOL is preferable over a silicone IOL. However, in the four studies identified by this review, we found insufficient evidence to support of HSM lenses over other unmodified lenses.
Regarding hydrophilic and hydrophobic acrylic IOLs, the largest RCT study in this review did not compare hydrophobic acrylic IOLs with hydrophilic acrylic IOLs (Alió 2002), and Roesel 2008 did not find any statistically significant differences at six months between hydrophobic and hydrophilic acrylic IOLs. However, a comparative case series by Abela-Formanek 2002 concluded that while all sharp-edged hydrophilic and hydrophobic IOLs performed well in uveitic eyes, acrylic hydrophilic IOLs achieved higher uveal biocompatibility compared with the acrylic hydrophobic IOLs.
Authors' Conclusions
Implications for practice
Cataract surgery with intraocular lens (IOL) implantation in people with quiescent inflammation for at least three months is no longer discouraged in people with uveitis. There is ethnic/geographic variation in the etiology of uveitis, but the limited results we have from the largest of the four studies suggest that the chance of improved visual outcome is higher and the risk of complication is lower when using hydrophobic acrylic rather than silicone lenses.
There is insufficient evidence that there are any differences in outcome or safety between hydrophobic and hydrophilic acrylic lenses; other than the one trial we identified, comparisons have been in nonrandomized studies (Abela-Formanek 2002). Two small studies in addition to Alió 2002 compared heparin-surface modification (HSM) poly(methyl methacrylate) (PMMA) IOLs with unmodified PMMA IOLs and found no clear differences; however, due to the small number of study participants, these studies may not have had enough participants in order to detect a difference. Based on the trials identified in this review, there is uncertainty as to which type of IOL provides the best visual and clinical outcomes in people with uveitis undergoing cataract surgery.
Implications for research
A large multicenter, international study would help answer the clinical questions of which types of people with uveitis fare better with cataract extraction and IOL implantation and which type of IOL to implant. Such study would require adequate numbers of each type of uveitic entity, and adults could be randomized to type of IOL using uveitic entity as a stratification variable for randomization. Subgroup analysis could then be performed on each uveitic entity. The outcome measures should be standard across all centers: classification standard (i.e. international Uveitis Study Group classification), uncorrected and best-corrected visual acuity (BCVA), anterior and posterior segment inflammation using the same grading system. Ideally, the postoperative observer would be masked to type of IOL when assessing visual acuity, inflammation, cystoid macular edema (CME), and posterior capsule opacification (PCO).
Plain Language Summary.
Comparison of artificial lenses placed in eyes with uveitis during cataract surgery
Background
Cataract, a cloudy lens within the eye, is a major complication in people with uveitis (inflammation of the middle layer of the eye). Different types of artificial lenses (intraocular lenses, IOLs) are available to insert into the eye after removal of the cloudy natural lens during cataract surgery. The purpose of this review is to summarize the effects of different artificial lenses in adults with uveitis.
Study characteristics
The review authors searched the medical literature up to August 2013 and identified four randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) containing 216 participants investigating different lens types.
Lens materials studied were acrylic, silicone, heparin surface modified, and poly(methyl methacrylate). The trials included participants with different causes of uveitis, compared different lens types, and reported different outcomes. Due to these differences across trials, no meta-analysis (pooling of trial data) was performed. The largest trial (140 participants) was funded by a professional society. The other three trials did not report their funding sources.
Key results
There is very limited evidence on which to determine the effects of different types of lens materials for people with uveitis. The results from the largest trial provide only preliminary evidence that acrylic lenses may perform better than silicone lenses in terms of improving vision and reducing the chances of post-surgical inflammation and complications. At this time, there is not enough data to conclude whether additional types of lenses are preferable to other types.
Quality of the evidence
Because the trials included small numbers of participants in each of the four lens-type groups, there is uncertainty in the results of the studies.
Acknowledgments
We acknowledge the assistance of Iris Gordon, Trials Search Coordinator, Cochrane Eyes and Vision Group (CEVG), with the design and execution of electronic searches for included studies. We also acknowledge the contributions of Dr. John Kempen during the development of the protocol and Ann Ervin and Elizabeth Ssemanda during the completion of the review. We thank Barbara Hawkins and Jenny Evans for providing comments to review drafts.
Richard Wormald (Co-ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Sources of Support: Internal sources:
No sources of support supplied
External sources:
-
National Eye Institute, National Institutes of Health, USA.
Cochrane Eyes and Vision Group US Project, Grant 1 U01 EY020522-01
Characteristics of Studies
Characteristics of included studies [ordered by study ID]
Alió 2002.
| Methods |
Study design: parallel-group RCT Number randomized: not reported Number analyzed: 140 participants Unit of analysis: individual (1 eye per participant) Length of follow-up: 1 year Sample size calculation: not reported |
|
| Participants |
Enrollment: 5-15 people enrolled at each study center (19 international centers) Gender: 64 (46%) men and 76 (54%) women were enrolled and completed the 1-year follow-up exam Age: ≥ 18 years Inclusion criteria: people with uveitis in good general health and willing to complete all required postoperative visits; uveitis in quiescence for 3 months; 18 years or older; and able to comprehend and sign a statement of informed consent Exclusion criteria: amblyopia; corneal dystrophy, scars, or vision-impairing disorders; congenital, traumatic, or rubella cataract; a congenitally abnormal cornea; diabetic retinopathy with macular compromise; macular edema; history of retinal detachment in past 6 months; uncontrolled glaucoma; microphthalmus; nonsighted or absent fellow eye; advanced or total optic atrophy; previous corneal transplant or ocular surgery; aniridia; burn-out uveitis; endothelial cell count less than 1500 cells/mm2; Fuchs cyclitis; juvenile rheumatoid uveitis; congenital abnormalities of the anterior segment; posterior uveitis not active in the anterior segment or major iridadenosis indicating large zonular dialysis; and intraoperative vitreous loss and vitrectomy, anterior chamber hyphema, posterior capsule tear, extensive zonular dehiscence or capsulotomy |
|
| Interventions |
4 lens types:
Not all study centers had access to each of the 4 study lenses; randomization was based on the availability of lenses at each center. In order to keep enrollment balanced among study centers, each center contributed a minimum of 5 participants and maximum of 15 participants |
|
| Outcomes | BCVA, postoperative inflammation, PCO relapses, and other clinical observations (i.e. corneal edema, cystoid macular edema, pupillary membrane crossing, posterior synechiae, giant and small cells on the IOL optic, pigment deposits, IOL decentration, PCO) Exams were performed at 1-2 days, 3-4 days, 6-8 days, 13-17 days, 27-33 days, 80-100 days (3 months), 170-190 days (6 months), and 11-13 months (1 year) |
|
| Notes |
Funding source: the International Ocular Inflammation Society Declarations of interest: “None of the authors or members of the International Ocular Inflammation Society has a financial or proprietary interest in any material or method mentioned” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was effected at the multicenter trial data coordination center (Alicante, Spain), in complete block design with IOL assignment in sequentially numbered, sealed envelopes” “Different causes made it impossible to ensure the availability of all lenses for all investigators; thus, each center had an individual randomized protocol for the available IOL following the same randomization code but excluding lenses that were not available. At least 3 of the 4 models were available at all institutions” |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by use of “sequentially numbered, sealed envelopes. Each patient entering the study was assigned to receive the lens implant designated for his or her patient entry number (e.g., patient #5 received the lens implant described on the randomization card in envelope #5)” |
| Masking (performance bias and detection bias) | High risk | The trial was unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Of the 170 patients considered for the study, 140 fulfilled the enrollment criteria and completed a 1-year follow-up” The study authors did not report whether there were enrolled participants who were lost to follow-up before 1 year (and thus not represented in this report) or if some method was used to account for missing data if lost before 1 year |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine (no study protocol available) |
| Other bias | Unclear risk | Insufficient information to determine |
Mester 1998.
| Methods |
Study design: intra-individual RCT Number randomized: 100 participants, 2 with uveitis and 98 without uveitis Number analyzed: 76 participants, 2 with uveitis and 74 without uveitis Unit of analysis: eye (1 eye of each participant randomized to receive an HSM PMMA IOL and the fellow eye to receive an unmodified PMMA IOL) Length of follow-up: 3 months Sample size calculation: not reported (only 2 of 100 total participants with uveitis) |
|
| Participants |
Enrollment: February 1994 to April 1995 in Sulzbach, Germany Gender: not reported Age: not reported Inclusion criteria: “at-risk” patients; predisposing risk factors for blood-aqueous barrier destabilization (including diabetes mellitus with or without retinopathy, glaucoma, pseudoexfoliation, and uveitis); and symmetrical cataract formation and identical surgical procedure in each eye by the same surgeon Number of participants in each risk group: diabetes mellitus with retinopathy (32) ; diabetes mellitus without retinopathy (26); glaucoma (26); pseudoexfoliation (14); uveitis (2) |
|
| Interventions |
2 lens types:
|
|
| Outcomes | The difference between postoperative and preoperative intraocular flare as a representative measure of surgically induced blood-aqueous barrier impairment Measurements were taken at 1 day preoperatively and at 1 day, 1 week, 6 weeks, and 3 months postoperatively |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Information regarding sequence generation, allocation concealment, masking were provided by the study investigators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used computer generated random list” (email communication with study investigators) |
| Allocation concealment (selection bias) | Low risk | “We used closed envelopes” (email communication with study investigators) |
| Masking (performance bias and detection bias) | Low risk | Participants “were not aware of the treatment assignments” (email communication with study investigators). Masking of study personnel and outcome assessors was not reported; however, the outcome measurement of intraocular flare was not likely to be influenced by lack of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no exclusions or losses to follow-up for the 2 participants with uveitis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine (no study protocol available) |
| Other bias | Unclear risk | Eyes of participants were operated on 6 weeks apart with the worse eye being operated on first. It was not reported whether the order was balanced with respect to which IOL type was implanted first |
Roesel 2008.
| Methods |
Study design: open-label, parallel-group RCT Number randomized: 60 participants (1 study center) Number analyzed: not reported Unit of analysis: individual (1 eye per participant) Length of follow-up: 6 months Sample size calculation: not reported |
|
| Participants |
Enrollment: August 2005 to February 2007 in Münster, Germany Gender: 18 (30%) men and 42 (70%) women Age: mean 51 years Inclusion criteria: people with chronic endogenous uveitis and clinically significant cataract Exclusion criteria: people with prior anterior segment surgery or other ocular pathology that would result in postoperative fibrin formation Inactivity of inflammation attempted for ≥ 3 months before surgery with introduction of topical and systemic corticosteroids and immunosuppressives |
|
| Interventions |
2 lens types:
All participants received endocapsular phacoemulsification surgery |
|
| Outcomes | BCVA (measured with Snellen chart), uveal biocompatibility (giant cells on IOL, anterior chamber cells, laser-flare photometry, and posterior synechiae), and capsular biocompatibility (PCO, number of Nd:YAG laser capsulotomies, and lens epithelial cell outgrowth) at 1, 3, and 6 months after surgery Adverse effects including endophthalmitis, elevated intraocular pressure, and macular edema also were reported |
|
| Notes |
Funding source: not reported Declarations of interest: “The authors have no financial interest in any of the materials used in this study” ClinicalTrials.gov Identifier: NCT00403832 Information regarding sequence generation, allocation concealment, masking were provided by the primary investigators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequences were computer generated (email communication with study investigators) |
| Allocation concealment (selection bias) | Low risk | Envelopes were used to conceal allocation (email communication with study investigators) |
| Masking (performance bias and detection bias) | High risk | Surgeons and outcome assessors unmasked since differences in IOL designs were obvious; data analyzers were masked to IOL type (email communication with study investigators). Masking of participants was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was no mention of exclusions or losses to follow-up in the manuscript, but the report of the number and percentage of eyes with visual acuity outcome of improvement of ≥ 2 lines BCVA suggest that there were losses/exclusions in both groups at 3 and 6 months (3 months: 4 eyes in the hydrophobic acrylic group and 1 eye in the hydrophilic acrylic group; 6 months: 5 eyes in the hydrophobic acrylic group and 6 eyes in the hydrophilic acrylic group) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine (no study protocol available) |
| Other bias | Unclear risk | Insufficient information to determine |
Tabbara 1998.
| Methods |
Study design: intra-individual RCT Number randomized: 25 participants, 14 with uveitis and 11 without uveitis (1 study center) Number analyzed: 22 participants, 11 with uveitis and 11 without uveitis Unit of analysis: eye (1 eye of each participant randomized to receive a HSM PMMA IOL and the fellow eye to receive an unmodified PMMA IOL) Length of follow-up: 24 months Sample size calculation: not reported (only 14 of 25 total participants with uveitis) |
|
| Participants |
Enrollment: April 1994 to March 1996 in Riyadh, Saudi Arabia Gender: 11 (44%) men and 14 (56%) women; 3 (21%) men and 11 (79%) women with uveitis Age: mean 46 years (range 11 to 81 years) for 14 participants with uveitis Inclusion criteria: people with either diabetes mellitus or inactive uveitis (no clinically detectable anterior segment inflammation for at least 3 months); ≥ 11 years of age; and bilateral cataract with bilateral similar inflammatory disease, but no active inflammation Exclusion criteria: glaucoma or evidence of active uveitis; diabetes with evidence of neovascularization or central retinal vein occlusion; and people with complications during surgery Number of participants in each group: inactive uveitis (14: 3 men, 11 women); type II diabetes mellitus (11:8 men, 3 women) |
|
| Interventions |
2 lens types:
Each IOL was placed in the capsular bag. All participants with uveitis underwent iridectomy at surgery |
|
| Outcomes |
Primary outcomes: cellular deposits on the surface of IOLs and posterior synechiae Secondary outcomes: visual acuity, intraocular pressure, anterior chamber cells and flare, and PCO Measurements were taken preoperatively and postoperatively at days 1 through 7; weeks 2, 3, and 4; and months 3, 6, 12, and 24. People with diabetes followed for 6 months and manuscript included 6-month outcomes only for people with uveitis Posterior synechiae, IOL cellular deposits, and posterior capsular fibrosis at 6 months reported in manuscript. 3 of 14 people with inactive uveitis missed the 6 month follow-up visit and were excluded from the report |
|
| Notes |
Funding source: lenses were provided by Pharmacia (Uppsala, Sweden) Declarations of interest: “The authors have no proprietary interest in any of the materials used in this study” Information regarding sequence generation, allocation concealment, masking, and missing patient data were provided by the primary investigators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random assignment was computer generated” (email communication with study investigators) |
| Allocation concealment (selection bias) | Low risk | “The intraocular lenses were in concealed in boxes and opened during the procedure by the operating room nurse” (email communication with study investigators) |
| Masking (performance bias and detection bias) | Low risk | “The surgeon was not aware of which eye had which IOL type until the study was completed”. The research coordinator and operating room nurses were not masked (email communication with study investigators). Masking of participants and outcome assessors was not reported; however, the 2 IOL types were similar in appearance and not likely to have influenced the outcome measurements |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 people with uveitis missed the 6-month follow-up visit and were not included in the final analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine (no study protocol available) |
| Other bias | Unclear risk | Insufficient information to determine |
BCVA: best-corrected visual acuity; HSM: heparin surface modified; IOL: intraocular lens; PCO: posterior capsule opacification; PMMA: poly(methyl methacrylate); RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abela-Formanek 2002 | Not an RCT, allocation of lens types by series (1: hydrophilic acrylic IOL; 2: hydrophobic acrylic IOL; 3: new-generation silicone) |
| Alio 1999 | Not an RCT (review of cataract surgery in eyes with uveitis) |
| Alio 2011 | Not an RCT (editorial on cataract surgery treatment options in eyes with uveitis) |
| Borgioli 1992 | Excluded people with uveitis |
| Brown 2011 | Not an RCT (prospective case series) |
| Chang 2010 | Not an RCT (review of IOL types; no coexisting cataract and uveitis) |
| Estafanous 2001 | Not an RCT (retrospective chart review) |
| Goel 2010 | Not an RCT (case report) |
| Jancevski 2010 | Not an RCT (review of management of people with coexisting cataract and uveitis) |
| Kanellopoulos 1995 | Not an RCT (case report) |
| Lai 1996 | Excluded people with uveitis |
| Levin 1996 | Not an RCT (general summary of recent advances in ophthalmology) |
| Martin 1992 | Excluded people with uveitis |
| Perry 2010 | Not an RCT (review of IOL use in people with uveitis) |
| Ríhová 1996 | Not an RCT (cohort of people with uveitis; 20/174 people had cataract surgery) |
| Tessler 1993 | Study authors did not specify the IOL type and did not respond to requests for information |
| Towler 1996 | Not an RCT (editorial comment on advances in ophthalmology) |
IOL: intraocular lens; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00000119.
| Methods | Randomized controlled trial |
| Participants | 80 people aged ≥ 18 years with uveitis who required cataract surgery; free of active inflammation for at least 3 months Exclusion criteria included corneal pathology or haze that precluded evaluation of the IOL, uncontrolled glaucoma, and diabetes mellitus. Monocular participants and those who could not complete 1 year of follow-up also excluded |
| Interventions | 1 eye randomly assigned to receive a heparin surface modified IOL or same model, but unmodified IOL |
| Outcomes | Primary outcome: development of cellular deposits on anterior surface of IOL at 1 year Secondary outcomes: visual acuity, development of synechiae, corneal endothelial cell counts, and intraocular inflammation |
| Notes | Sponsor: National Eye Institute (NEI) ClinicalTrials.gov Identifier: NCT00000119. Study status listed as unknown in clinicaltrials.gov as 8 November 2013 We contacted the Office of Communication, Health Education, and Public Liaison at NEI for information about the study. NEI did not reply with additional information for this trial |
NCT00001311.
| Methods | Randomized controlled trial |
| Participants | People with uveitis who required cataract surgery; free of active inflammation for at least 3 months |
| Interventions | People randomly assigned to receive a standard IOL or heparin surface modified IOL |
| Outcomes | Periodic follow-up visits for about 1 year may have included: fluorescein angiography to evaluate the blood vessels of the retina; specular microscopy to examine the surface of the IOL; cell and flare measurements to evaluate inflammation, and ultrasound to examine the back of the eye |
| Notes | Sponsor: National Eye Institute (NEI) intramural research (Study ID: 92-EI-0157) ClinicalTrials.gov Identifier: NCT00001311 We contacted the Office of Communication, Health Education, and Public Liaison at NEI for information about the study. NEI replied via email that the principal investigator on the trial, Dr. Scott Whitcup, left the NEI. Our attempts to contact Dr. Scott Whitcup did not yield further information |
IOL: intraocular lens.
Data and Analyses
This review has no analyses.
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Uveitis
#2 uveiti*
#3 MeSH descriptor Iritis
#4 iritis
#5 MeSH descriptor Iridocyclitis
#6 iridocyclitis
#7 MeSH descriptor Pars Planitis
#8 pars planitis
#9 MeSH descriptor Choroiditis
#10 retinochoroidit* or choroidit*
#11 MeSH descriptor Behcet Syndrome
#12 MeSH descriptor Uveomeningoencephalitic Syndrome
#13 bechet or Vogt-Koyanagi-Harada or fuch
#14 MeSH descriptor Retinitis
#15 retinitis
#16 MeSH descriptor Ophthalmia, Sympathetic
#17 ophthalmia* sympathetic
#18 MeSH descriptor Arthritis, Juvenile Rheumatoid
#19 juvenile near/2 rheumatoid near/2 arthriti*
#20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
#21 MeSH descriptor Cataract
#22 MeSH descriptor Cataract Extraction
#23 MeSH descriptor Phacoemulsification
#24 MeSH descriptor Capsulorhexis
#25 (cataract* or phacoemulsifcat*)
#26 (#21 OR #22 OR #23 OR #24 OR #25)
#27 MeSH descriptor Lens Implantation, Intraocular
#28 MeSH descriptor Lenses, Intraocular
#29 MeSH descriptor Acrylic Resins
#30 MeSH descriptor Coated Materials, Biocompatible
#31 MeSH descriptor Polymethyl Methacrylate
#32 MeSH descriptor Silicone Elastomers
#33 MeSH descriptor Heparin
#34 (intraocular lens* or IOL*)
#35 lens near/20 (acrylic or silicone or polymethylmethacrylate or PMMA)
#36 IOL* near/20 (acrylic or silicone or polymethylmethacrylate or PMMA)
#37 heparin
#38 (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37)
#39 (#20 AND #26 AND #38)
Appendix 2. MEDLINE (OvidSP) search strategy
randomized controlled trial.pt.
(randomized or randomized).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1-7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp uveitis/
uveiti$.tw.
exp iritis/
iritis.tw.
exp iridocyclitis/
iridocyclitis.tw.
exp pars planitis/
pars planitis.tw.
exp choroiditis/
(retinochoroidit$ or choroidit$).tw.
exp behcet syndrome/
exp Uveomeningoencephalitic Syndrome/
(bechet or Vogt-Koyanagi-Harada or fuch).tw.
exp retinitis/
retinitis.tw.
exp ophthalmia, sympathetic/
(ophthalm$ adj2 sympathetic).tw.
exp arthritis juvenile rheumatoid/
(juvenile adj2 rheumatoid adj2 arthriti$).tw.
or/13-31
exp cataract/
exp cataract extraction/
exp phacoemulsification/
exp capsulorhexis/
(cataract$ or phacoemulsificat$).tw.
or/33-37
exp lens implantation intraocular/
exp lenses intraocular/
exp acrylic resins/
exp coated materials, biocompatible/
exp polymethylmethacrylate/
exp silicone elastomers/
exp heparin/
(intraocular lens$ or IOL$).tw.
((acrylic or silicone or polymethylmethacrylate or PMMA) adj20 lens$).tw.
((acrylic or silicone or polymethylmethacrylate or PMMA) adj20 IOL$).tw.
heparin.tw.
or/39-49
32 and 38 and 50
12 and 51
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
exp randomised controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1-5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12-21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25-28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp uveitis/
uveiti$.tw.
exp iritis/
iritis.tw.
exp iridocyclitis/
iridocyclitis.tw.
exp intermediate uveitis/
pars planitis.tw.
exp choroiditis/
(retinochoroidit$ or choroidit$).tw.
exp behcet disease/
exp Meningoencephalitis/
(bechet or Vogt-Koyanagi-Harada or fuch).tw.
exp retinitis/
retinitis.tw.
exp sympathetic ophthalmia/
(ophthalm$ adj2 sympathetic).tw.
exp Juvenile Rheumatoid Arthritis/
(juvenile adj2 rheumatoid adj2 arthriti$).tw.
or/33-51
exp cataract/
exp cataract extraction/
exp phacoemulsification/
exp capsulorhexis/
(cataract$ or phacoemulsificat$).tw.
or/53-57
exp lens implantation/
exp lens implant/
exp acrylic acid resin/
exp biomaterial/
exp polymethylmethacrylate/
exp silastic/
exp heparin/
(intraocular lens$ or IOL$).tw.
((acrylic or silicone or polymethylmethacrylate or PMMA) adj20 lens$).tw.
((acrylic or silicone or polymethylmethacrylate or PMMA) adj20 IOL$).tw.
heparin.tw.
or/59-69
52 and 58 and 70
32 and 71
Appendix 4. LILACS search strategy
uveiti$ or iritis or retinitis and cataract$ or phacoemulsif$ or capsulorhexis and lens$ or intraocular or IOL$ or acrylic or silicone or polymethylmethacrylate or PMMA
Appendix 5. metaRegister of Controlled Trials search strategy
uveitis and cataract
Appendix 6. ClinicalTrials.gov search strategy
Uveitis AND Cataract
Appendix 7. ICTRP search strategy
Uveitis AND Cataract
Footnotes
Indicates the major publication for the study
Contributions of Authors: Conceiving the review: Ann Ervin (AE), John Kempen (JK)
Designing the review: AE, JK, Elizabeth Ssemanda (ES), KL
Coordinating the review: AE, ES, KL
- Designing search strategies: Iris Gordon (Cochrane Eyes and Vision Group), AE, JK, ES, KL
- Undertaking searches: Iris Gordon (Cochrane Eyes and Vision Group)
- Screening search results: AE, ES, KL, TL
- Organizing retrieval of papers: AE, ES, KL
- Screening retrieved papers against inclusion criteria: AE, ES, KL, TL
- Appraising quality of papers: AE, ES, KL
- Extracting data from papers: AE, ES, KL
- Writing to authors of papers for additional information: AE, ES, KL
- Providing additional data about papers: AE, IK, ES, KL
- Obtaining and screening data on unpublished studies: AE, ES, KL
- Entering data into RevMan: AE, ES, KL
Analysis of data: AE, ES, KL
- Providing a methodological perspective: AE, ES, KL
- Providing a clinical perspective: IK, TL
- Providing a policy perspective: IK, TL
- Providing a consumer perspective: IK
Writing the review: AE, IK, ES, KL, TL
Providing general advice on the review: AE, ES, KL, IK, TL
Securing funding for the review: N/A
Performing previous work that was the foundation of the current study: N/A
Guarantor of the review: IK, KL
Declarations of Interest: None known.
Differences Between Protocol and Review: The World Health Organization's International Clinical Trials Registry Platform was searched to identify potential inclusions from other international trial registries. We added two patient-important visual outcomes (BCVA 20/40 or better and BCVA 20/200 or worse, Snellen equivalent) and visual acuity as a continuous outcome (measured as mean difference) for this review.
Notes: We contacted the National Eye Institute (NCT00001311; NCT00000119), Alió 2002, Mester 1998, Roesel 2008, Tabbara 1998, and Tessler 1993 to pose additional methodologic and outcome-specific queries.
References to studies included in this review
- *.Alió JL, Chipont E, BenEzra D, Fakhry MA International Ocular Inflammation Society, Study Group of Uveitic Cataract Surgery. Comparative performance of intraocular lenses in eyes with cataract and uveitis. Journal of Cataract and Refractive Surgery. 2002;28(12):2096–108. doi: 10.1016/s0886-3350(02)01452-9. [DOI] [PubMed] [Google Scholar]
- Alió JL, Chipont EM, Fakhry MA, Ezra DB. Comparative performance of intraocular lenses in uveitic eyes with cataract. American Academy of Ophthalmology. 2000;113 [Google Scholar]
- Burtin T. Comparison of heparin surface-modified and soft acrylic hydrophobic intraocular lenses in chronic uveitis. American Academy of Ophthalmology. 1999;178 [Google Scholar]
- Chipont E, Alió JL, Smith AF, Fakhry MA, Garcia P. Comparative performance of four types of intraocular lenses in the uveitic eye: health economic analysis. American Academy of Ophthalmology. 2002;240 [Google Scholar]
- Papaliodis GN, Nguyen QD, Samson CM, Foster CS. Intraocular lens tolerance in surgery for cataracta complicata: assessment of four implant materials. Seminars in Ophthalmology. 2002;17(3-4):120–3. doi: 10.1076/soph.17.3.120.14788. [DOI] [PubMed] [Google Scholar]
- Mester U, Strauss M, Grewing R. Biocompatibility and blood-aqueous barrier impairment in at-risk eyes with heparin-surface-modified or unmodified lenses. Journal of Cataract and Refractive Surgery. 1998;24(3):380–4. doi: 10.1016/s0886-3350(98)80327-1. [DOI] [PubMed] [Google Scholar]
- Heinz C, Roesel M, Heiligenhaus A. Uveal and capsular biocompatibility of two acrylic intraocular lenses in patients with non-infectious uveitis. Investigative Ophthalmology and Visual Science. 2007;48 doi: 10.1007/s00417-008-0886-4. ARVO E-Abstract 3915. [DOI] [PubMed] [Google Scholar]
- Roesel M, Heinz C, Heimes B, Koch JM, Heiligenhaus A. Uveal and capsular biocompatibility of two foldable acrylic intraocular lenses in patients with endogenous uveitis - a prospective randomized study. Graefe's Archive for Clinical and Experimental Ophthalmolog y. 2008;246(11):1609–15. doi: 10.1007/s00417-008-0886-4. [DOI] [PubMed] [Google Scholar]
- Roesel M, Heinz C, Koch JM, Heiligenhaus A. Cataract surgery in uveitis. Ophthalmology. 2008;115(8):1431. doi: 10.1016/j.ophtha.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Tabbara KF, Al-Kaff AS, Al-Rajhi AA, Al-Mansouri SM, Badr IA, Chavis PS, et al. Heparin surface-modified intraocular lenses in patients with inactive uveitis or diabetes. Ophthalmology. 1998;105(5):843–5. doi: 10.1016/S0161-6420(98)95023-0. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- *.Abela-Formanek C, Amon M, Schauersberger J, Kruger A, Nepp J, Schild G. Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract: comparison to a control group. Journal of Cataract and Refractive Surgery. 2002;28(7):1141–52. doi: 10.1016/s0886-3350(02)01425-6. [DOI] [PubMed] [Google Scholar]
- Abela-Formanek C, Amon M, Schauersberger J, Schild G, Kolodjaschna J, Barisani-Asenbauer T, et al. Uveal and capsular biocompatibility of 2 foldable acrylic intraocular lenses in patients with uveitis or pseudoexfoliation syndrome: comparison to a control group. Journal of Cataract and Refractive Surgery. 2002;28(7):1160–72. doi: 10.1016/s0886-3350(02)01360-3. [DOI] [PubMed] [Google Scholar]
- Abela-Formanek C, Amon M, Schild G, Schauersberger J, Kolodjaschna J, Barisani-Asenbaum T, et al. Inflammation after implantation of hydrophilic acrylic, hydrophobic acrylic, or silicone intraocular lenses in eyes with cataract and uveitis: comparison to a control group. Journal of Cataract and Refractive Surgery. 2002;28(7):1153–9. doi: 10.1016/s0886-3350(02)01321-4. [DOI] [PubMed] [Google Scholar]
- Alio JL, Chipont E. Surgery of cataract in patients with uveitis. Developments in Ophthalmology. 1999;31:166–74. doi: 10.1159/000060764. [DOI] [PubMed] [Google Scholar]
- Alio JL. August consultation #4. Journal of Cataract and Refractive Surgery. 2011;37(8):1563–4. [Google Scholar]
- Borgioli M, Coster DJ, Fan RF, Henderson J, Jacobi KW, Kirkby GR, et al. Effect of heparin surface modification of polymethylmethacrylate intraocular lenses on signs of postoperative inflammation after extracapsular cataract extraction. One-year results of a double-masked multicenter study. Ophthalmology. 1992;99(8):1248–54. doi: 10.1016/s0161-6420(92)31816-0. [DOI] [PubMed] [Google Scholar]
- Brown DC, Gills JP, 3rd, Trattler WB, Newsom TH, Weinstock RJ, Sanders DR. Prospective multicenter trial assessing effectiveness, refractive predictability and safety of a new aberration free, bi-aspheric intraocular lens. Contact Lens and Anterior Eye. 2011;34(4):188–92. doi: 10.1016/j.clae.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Chang GC, Pineda R. Phakic intraocular lenses. International Ophthalmology Clinics. 2010;50(1):119–28. doi: 10.1097/IIO.0b013e3181c55227. [DOI] [PubMed] [Google Scholar]
- Estafanous MF, Lowder CY, Meisler DM, Chauhan R. Phacoemulsification cataract extraction and posterior chamber lens implantation in patients with uveitis. American Journal of Ophthalmology. 2001;131(5):620–5. doi: 10.1016/s0002-9394(00)00909-0. [DOI] [PubMed] [Google Scholar]
- Goel R, Thangkhiew L, Yadava U, Kumar S. Management of bilateral idiopathic healed sclerokeratouveitis with ciliary and intercalary staphyloma with complicated cataract and secondary glaucoma. Indian Journal of Ophthalmology. 2010;58(5):444–5. doi: 10.4103/0301-4738.67043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancevski M, Foster CS. Cataracts and uveitis. Current Opinion in Ophthalmology. 2010;21(1):10–4. doi: 10.1097/ICU.0b013e328332f575. [DOI] [PubMed] [Google Scholar]
- Jancevski M, Foster CS. Cataracts and uveitis. Discovery Medicine. 2010;9(44):51–4. [PubMed] [Google Scholar]
- Kanellopoulos AJ, Weintraub J, Rahn EK. Phacoemulsification and silicone foldable intraocular lens implantation in a patient with chronic sarcoid uveitis. Journal of Cataract and Refractive Surgery. 1995;21(4):364–5. doi: 10.1016/s0886-3350(13)80519-6. [DOI] [PubMed] [Google Scholar]
- Lai YK, Fan RF. Effect of heparin-surface-modified poly(methyl methacrylate) intraocular lenses on the postoperative inflammation in an Asian population. Journal of Cataract and Refractive Surgery. 1996;22(Suppl 1):830–4. doi: 10.1016/s0886-3350(96)80170-2. [DOI] [PubMed] [Google Scholar]
- Levin LA. Ophthalmology. JAMA. 1996;275(23):1834–6. [PubMed] [Google Scholar]
- Martin RG, Sanders DR. Visual, astigmatic, and inflammatory results with the Staar AA-4203 single-piece foldable IOL: a randomized, prospective study. Ophthalmic Surgery. 1992;23(11):770–5. [PubMed] [Google Scholar]
- Perry LJ, Papaliodis GN. Selection of intraocular lenses in patients with uveitis. International Ophthalmology Clinics. 2010;50(1):61–70. doi: 10.1097/IIO.0b013e3181c55503. [DOI] [PubMed] [Google Scholar]
- Ríhová E, Havlíková M, Michalová K, Boguszaková J, Stara J, Poch T. Cataract surgery in patients with endogenous uveitis [Operace katarakty u pacientu s endogennì uveitidou] Ceska a Slovenska Oftalmologie. 1996;52(2):98–103. [PubMed] [Google Scholar]
- Tessler HH, Farber MD. Intraocular lens implantation versus no intraocular lens implantation in patients with chronic iridocyclitis and pars planitis. A randomized prospective study Ophthalmology. 1993;100(8):1206–9. doi: 10.1016/s0161-6420(93)31504-6. [DOI] [PubMed] [Google Scholar]
- Tessler HH, Farber MD. Intraocular lens implants in patients with uveitis. Ophthalmology. 1990;97(12):1579. doi: 10.1016/s0161-6420(90)38022-x. [DOI] [PubMed] [Google Scholar]
- Towler HM, Lightman S. Ophthalmology. BMJ. 1996;312(7035):889–92. doi: 10.1136/bmj.312.7035.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
- NCT00000119. Safety and efficacy of a heparin-coated intraocular lens in uveitis. [accessed 27 February 2014]; clinicaltrials.gov/show/NCT00000119.
- NCT00001311. Modified intraocular lens to reduce eye inflammation after cataract surgery in uveitis patients. [accessed 27 February 2014]; clinicaltrials.gov/ct2/show/study/NCT00001311.
Additional references
- Alió JL, Chipont E. Phacoemulsification in patients with uveitis. In: Lu LW, Fine IH, editors. Phacoemulsification in Difficult and Challenging Cases. New York: Thieme; 1999. pp. 65–74. [Google Scholar]
- Bellucci R. An introduction to intraocular lenses: material, optics, haptics, design and aberration. In: Güell JL, editor. Cataract ESASO Course Series. Vol. 3. Basel: Karger; 2013. pp. 38–55. [Google Scholar]
- Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, et al. Chronic severe uveitis: aetiology and visual outcomes in 927 patients from a single centre. Medicine. 2001;80(4):263–70. doi: 10.1097/00005792-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Chapter 9: Analysing data and undertaking meta-analyses. updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Dharmaraj S, Azar N. Controversies of implanting intraocular lenses in infancy. International Ophthalmology Clinics. 2005;45(4):61–81. doi: 10.1097/01.iio.0000176371.39629.81. [DOI] [PubMed] [Google Scholar]
- Dunn JP. Cataract surgery in patients with uveitic cataract. Techniques in Ophthalmology. 2009;7(1):3–7. [Google Scholar]
- Foster RE, Lowder CY, Meisler DM, Zakov ZN. Extracapsular cataract extraction and posterior chamber intraocular lens implantation in uveitis patients. Ophthalmology. 1992;99(8):1234–41. doi: 10.1016/s0161-6420(92)31818-4. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Chapter 8: Assessing risk of bias in included studies. updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Hiles DA, Watson BA. Complications of implant surgery in children. Journal - American Intra-Ocular Implant Society Journal. 1979;5(1):24–32. doi: 10.1016/s0146-2776(79)80010-5. [DOI] [PubMed] [Google Scholar]
- Kang S, Kim MJ, Park SH, Joo CK. Comparison of clinical results between heparin surface modified hydrophilic acrylic and hydrophobic acrylic intraocular lens. European Journal of Ophthalmology. 2008;18(3):377–83. doi: 10.1177/112067210801800311. [DOI] [PubMed] [Google Scholar]
- Lichter PR. Intraocular lenses in uveitis patients. Ophthalmology. 1989;96(3):279–80. doi: 10.1016/s0161-6420(89)32894-6. [DOI] [PubMed] [Google Scholar]
- McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. American Journal of Ophthalmology. 1996;121(1):35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández S, Martín-Mola E. Uveitis. Best Practice Research in Clinical Rheumatology. 2006;20(3):487–505. doi: 10.1016/j.berh.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology. 1999;106(4):710–22. doi: 10.1016/S0161-6420(99)90155-0. [DOI] [PubMed] [Google Scholar]
- Reeves SW, Sloan FA, Lee PP, Jaffe GJ. Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006;113(2):302–7. doi: 10.1016/j.ophtha.2005.10.008. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. [Google Scholar]
- Rojas B, Foster CS. Cataract surgery in patients with uveitis. Current Opinion in Ophthalmology. 1996;7(1):11–6. doi: 10.1097/00055735-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Rojas B, Zafirakis P, Foster CS. Cataract surgery in patients with uveitis. Current Opinion in Ophthalmology. 1997;8(1):6–12. [PubMed] [Google Scholar]
- Trivedi RH, Wilson ME, Jr, Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. Journal of AAPOS. 2006;10(2):117–23. doi: 10.1016/j.jaapos.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Van Gelder RN, Leveque TK. Cataract surgery in the setting of uveitis. Current Opinion in Ophthalmology. 2009;20(1):42–5. doi: 10.1097/ICU.0b013e32831b9b22. [DOI] [PubMed] [Google Scholar]
- Woodcock M, Shah S, Smith RJ. Recent advances in customising cataract surgery. BMJ. 2004;328(7431):92–6. doi: 10.1136/bmj.328.7431.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Ssemanda E, Lindsley K, Ervin AM, Kempen J. Comparison of intraocular lens types for cataract surgery in eyes with uveitis. Cochrane Database of Systematic Reviews. 2008;3 doi: 10.1002/14651858.CD007284. [DOI] [PMC free article] [PubMed] [Google Scholar]


