Abstract
Mucinous tumors of the ovary represent a spectrum of neoplastic disorders, including benign mucinous cystadenoma, pseudomyxoma peritonei, mucinous tumors of low malignant potential (borderline), and invasive mucinous ovarian carcinoma. These tumors are related closely to each other and are distinct from other histologic subtypes of epithelial ovarian neoplasms from a clinical, histologic, and molecular standpoint. A continuum appears to be present from benign to borderline to malignant, which is different from other types of epithelial ovarian cancer. Mutational profiles are also distinct, as KRAS mutations are common, but p53 and BRCA mutations are infrequent. These characteristics lead to specific biologic behavior and guide both clinical management and research efforts in patients with mucinous ovarian tumors.
Keywords: Mucinous, Mucinous tumor, Gynecologic cancers, Gynecologic cancer, Ovary, Carcinoma, Tumor, Borderline, Pseudomyxoma, Neoplasm, Mucinous ovarian tumors, Oncology
Introduction
Historically, all epithelial ovarian cancers have been treated in a similar fashion, with upfront debulking surgery, staging and/or tumor reduction, and adjuvant chemotherapy for all but early stage disease, usually with a taxane and a platinum agent. Clinical decision making and prognostic information have been determined by pooling aggregate data from all epithelial cancer subtypes, including serous, mucinous, endometrioid, clear cell, Brenner, mixed, and undifferentiated histologies. Mucinous ovarian carcinoma (mOC), however, represents less than five percent of all epithelial ovarian malignancies, and, until recently, the subtleties of this histologic subtype were lost in the larger framework of investigating epithelial ovarian cancers as a whole [1].
Beginning in 2004, however, the profile of mOC began to emerge as a separate entity, with a specific clinical presentation and biological behavioral pattern [2]. It appeared that outcomes were worse when mOCs were compared with all other epithelial ovarian cancers, leading to further research and a body of work that has shown mucinous tumors of the ovary to be quite distinct from other forms of epithelial ovarian neoplasms. This article will examine the epidemiology, clinical presentation, biologic behavior, pathologic characteristics, molecular signatures, and research efforts aimed at better understanding and improving the outcomes for women with mucinous ovarian tumors.
Epidemiology
Mucinous tumors represent a spectrum of malignant behavior, and have benign, borderline, and invasive histologic variants. Among benign ovarian neoplasms, mucinous cystadenomas account for approximately 10–15 % of all cases [3, 4]. Borderline tumors, or tumors of low malignant potential (LMP tumors), may be more common than invasive primary mucinous ovarian carcinomas, and comprise up to 67 % of mucinous neoplasms that are not considered strictly benign [5]. Pathologic criteria are crucial in making the correct diagnosis, and classification systems have been an area of debate, so making these epidemiologic determinations has been difficult [6].
Each year, the projected United States statistics for incidence and mortality of specific malignancies are released. For 2013, the projected incidence of ovarian cancer is 22,240 cases and the projected mortality is 14,030 cases. However, this comprises all ovarian malignancies, including epithelial, germ cell, and sex cordstromal tumors, and does not specify the subtype of histology within the heading of ovarian cancer [7]. Estimates of the frequency of primary mOC range from 6–25 %, but a systematic review to exclude tumors of low malignant potential and metastatic lesions from gastrointestinal, pancreatic, or other gynecologic primary tumors suggests that the true percentage of ovarian carcinomas represented by primary mucinous tumors is substantially less, at 2.4 % [8]. Another study by Shimada et al. [9] corroborated these findings, and a careful pathologic review of 1,400 ovarian cancer cases resulted in reclassification of most patients initially thought to have primary invasive mucinous ovarian cancer; this percentage declined from 16 % to 4.9 %, with the remainder reclassified as having mucinous intraepithelial carcinoma, borderline tumor, or metastatic disease from a non-ovarian origin.
This low frequency is confirmed by evaluating the percentage enrollment on clinical trials represented by mucinous carcinomas of the ovary. For example, in Gynecologic Oncology Group (GOG) trial 111 (cisplatin and cyclophosphamide vs. cisplatin and paclitaxel), only 3.4 % of the patients enrolled (14 of 410) had mucinous tumors [10]. In intergroup trial IV-10 (cisplatin and cyclophosphamide vs. cisplatin and paclitaxel), 4.4 % of the patients enrolled (30 of 680) had mucinous tumors [11]. Subsequently, GOG trial 132 (cisplatin vs. paclitaxel vs. cisplatin and paclitaxel) enrolled 2.6 % of the patients (16 of 614) with mucinous tumors [12]. Finally, GOG trial 182 compared standard-of-care paclitaxel and carboplatin to four other platinum-based regimens as adjuvant chemotherapy in women with stage III or IV epithelial ovarian cancer. Of 4312 patients enrolled with primary epithelial ovarian cancer, only 1.6 % (71 of 4312) had primary mOC [13].
Despite the low percentage of patients with epithelial ovarian cancer who have a primary mOC, the conclusions drawn from these randomized studies have been applied to mOCs until recently. This phenomenon is problematic for all rare tumors, as rare tumors are lumped together with more common tumors regardless of real differences. While this would be acceptable if the clinical behavior and treatment response mirrored more common histologies, they do not, and should be studied separately and treated differently [1].
Clinical Presentation
The clinical features of mucinous ovarian neoplasms are distinct from their other epithelial counterparts, and certain characteristics are typical for primary mucinous ovarian neoplasms. These include symptoms at presentation, rate of bilaterality, stage at diagnosis, potential for lymphatic dissemination, and serum tumor markers; such characteristics have lead to specific surgical recommendations.
Symptoms at Presentation
Mucinous cystadenomas usually occur as a large, multiloculated cystic mass with mucus-containing fluid [14]. These tumors occur most commonly in women in their twenties to forties, but occurrences in adolescent and even premenarchal girls, as well as postmenopausal patients, have been documented [15]. The mean size at presentation is 18 cm, and mucinous tumors can become extremely large and fill the entire abdominopelvic cavity, occasionally presenting with ureteral obstruction or abdominal compartment syndrome [16–18]. The large size can itself sometimes suggest a mucinous histology.
When determining the primary or metastatic nature of the mucinous neoplasm, size and laterality can suggest the tumor origin, as primary tumors tend to be larger and unilateral, compared with metastatic lesions. The mean size of primary mOCs has been documented as 16–20 cm (range, 5–48 cm), compared with 11–12 cm (range, 2–24 cm) for metastatic cancers. This is not pathognomonic, however, as 32–48 % of metastatic tumors are over 10 cm [8, 19].
Rate of Bilaterality
Most mucinous tumors are unilateral, especially when primarily ovarian in origin. A large retrospective series and a SEER database analysis have both shown that 79 % of mucinous tumors are unilateral [16, 20]. In the SEER database analysis, 21.3 % of women with primary mOCs had bilateral involvement (355 of 1,665 women), significantly less than in serous ovarian cancers, in which 57.5 % of women had bilateral involvement (4,289 of 7,453 women). Similarly, borderline mucinous ovarian tumors were less likely bilateral (7 %) than their serous borderline counterparts (29.8 %) [20].
When comparing primary versus metastatic mucinous carcinomas, primary mOCs were much less likely to be bilateral (0–17 %) than mucinous tumors metastatic to the ovary (75–77 %) [8, 19, 21]. Based on these findings of large, unilateral tumors favoring a primary ovarian origin, Seidman et al., has proposed an algorithm incorporating these two factors. In their series, a unilateral tumor greater than 10 cm correctly predicted primary ovarian origin in 82 % of cases. In patients with bilateral tumors less than 10 cm, metastatic disease was correctly predicted in 95 % of cases [8]. Others have since validated this approach [21].
Stage at Diagnosis
In contrast to serous ovarian carcinomas, in which only 4 % of patients are stage I at diagnosis, 83 % of mOCs are stage I at the time of diagnosis [22]. Thus, only 17 % of patients with mOCs are stage II or higher.
Potential for Lymphatic Dissemination
Multiple authors have confirmed that a mucinous tumor grossly limited to the ovary will not have occult lymph node metastasis. Of 146 total patients in three series with borderline or invasive mOC that appeared to be confined to the ovary at the time of surgery, none had lymphatic metastases [23–25]. All of these patients underwent lymphadenectomy as part of their staging procedures, and none were found to have lymph node disease. In contrast, 10 % of patients with apparent stage I serous carcinoma of the ovary have been reported to have occult nodal metastasis at the time of diagnosis [26]. Therefore, it is not necessary to perform pelvic and/or para-aortic lymphadenectomy as part of the staging procedure in patients with primary mOC grossly limited to the ovary [25].
Tumor Markers
Carcinoembryonic antigen (CEA) is the most useful serum tumor marker to identify mOC preoperatively and to follow the progress of a patient with mOC post-operatively. Though it is widely used in the detection and surveillance of gastrointestinal carcinomas, CEA is elevated in almost one third of all ovarian carcinomas. It is much more likely to be elevated in mOCs than in nonmucinous ovarian carcinomas (88 % vs. 19 %) [27, 28].
Other biomarkers have been investigated as potentially useful in the differentiation of mucinous from other types of epithelial ovarian cancers. Immunoassay evaluation of 58 serum biomarkers in patients with serous, mucinous, clear cell, and endometrioid ovarian carcinomas revealed significant differences in levels between serous and mucinous ovarian carcinomas in ten biomarkers. While serous tumors had elevated CA125, follicle-stimulating hormone, luteinizing hormone, and SMRP levels, mucinous tumors had higher levels of CA72-4, matrix metalloproteinase-9, CD40L, insulin-like growth factor-binding protein-1, myeloperoxidase, and tissue plasminogen activator-1 [29].
Surgical Recommendations
The gold standard for the treatment of any suspected ovarian mass includes intact removal of the involved adnexa with intraoperative pathology evaluation [30–32]. This has historically implied laparotomy, total hysterectomy, bilateral salpingo-oophorectomy, and staging procedure including lymphadenectomy. There are many nuances to the appropriate surgical management of the patient with a mucinous ovarian tumor, and close inspection of the upper and lower gastrointestinal tract should always be performed in the event of a suspected primary mOC, as primary tumors are relatively rare [32].
Since most mucinous tumors of the ovary are large, most surgeons will perform an exploratory laparotomy with removal of the involved adnexa. If the patient is post-menopausal, a total hysterectomy and bilateral salpingo-oophorectomy may be considered regardless of the histology. However, many patients are premenopausal, and the unilateral nature of most mucinous tumors, whether benign, borderline, or invasive primary ovarian cancers, allow fertility preservation with conservation of the normal appearing uterus and contralateral ovary in apparent early stage disease [14, 32–35]. Since most patients do have early stage disease, this is not an uncommon situation.
When the surgeon enters the abdomen, care should be taken to remove the involved ovary intact without spillage of the mucinous contents, as rupture of a stage I mOC may increase its potential for recurrence [36]. Benign mucinous ovarian cystadenomas are by definition confined to the ovary, and no further procedure is required. While appendectomy was previously performed for any ovarian tumor with mucinous histology, including benign lesions, current data support not performing an appendectomy as long as the appendix appears normal and there is no evidence of pseudomyxoma peritonei [37].
The abdomen and pelvis should be meticulously explored and any abnormal area should be noted. If the tumor appears to be widespread, the surgeon should determine whether the disseminated disease is resectable, either to a level of less than one centimeter - historically considered “optimal” - or preferably to no gross residual disease left at the completion of surgery. If this is possible, then every effort should be made for maximal debulking surgery; widespread disease does mandate removal of the contralateral ovary and the uterus. If total gross surgical resection is not possible, then consideration should be given to alleviating patient symptoms, e.g., bowel resection for an impending obstruction, and stopping the procedure in favor of chemotherapy [32, 38].
Intraoperative determination of the exact degree of pathologic abnormality is difficult, as these tumors are often quite large, intraoperative sectioning is by definition limited to a few areas of the tumor, and the differences between benign, borderline, and invasive carcinomas are subtle. Additionally, the determination of a primary versus metastastic mucinous tumor can be difficult [1, 39]. Therefore, the surgeon is often faced with making decisions about the extent of surgery with incomplete knowledge of the tumor histology. Since these tumors are often early stage and unilateral, a patient in her reproductive years with disease apparently confined to one ovary can undergo fertility-sparing surgery with no adverse effect on her prognosis [33–35]. Every patient with either borderline or invasive components identified, however, should undergo a staging procedure to exclude the possibility of occult extraovarian disease, as this would change the prognosis and recommended adjuvant therapy. Staging in patients with mucinous tumors includes a thorough evaluation of the peritoneal cavity with sampling of any suspicious areas, pelvic washings, peritoneal biopsies, and infracolic omentectomy [32]. Sampling of the pelvic and para-aortic lymph nodes is not warranted and should not be considered as part of the staging procedure, based on the zero incidence of nodal metastases in these patients. Any enlarged lymph node, however, should be sampled [25]. It should be noted that the need for staging in patients with borderline mucinous tumors is controversial [40]; however, given the difficulty of definitively excluding invasion at the time of intraoperative pathologic evaluation and the substantial likelihood of a permanent diagnosis being rendered as invasive disease, staging without lymph node sampling appears to be a low risk procedure with the potential to avoid another operation pending a final diagnosis of invasive disease.
As the use of minimally invasive surgery has increased and the scope of indications has broadened, minimally invasive surgery has been increasingly used for the management of patients with mucinous ovarian tumors, even when they are quite large. Multiple reports exist of the safe and feasible removal of mucinous tumors laparoscopically, with several techniques reported to effect removal without spillage of the cyst contents [41, 42]. Morcellation in the peritoneal cavity or contamination of the peritoneal cavity and/or trocar sites should be absolutely avoided. The mass, once detached, should be placed into an intraperitoneal specimen bag, and the edges of the bag drawn up through one abdominal incision, which can be enlarged. Once the mass is in this way isolated from the skin, subcutaneous tissues, and peritoneal cavity by its placement in the bag, it can be drained with a large bore spinal needle attached to a syringe or with a suction device without risking peritoneal contamination. Alternatively, a laparoscopic needle can be used to decompress a cyst, which is thought to be benign, to allow its placement into a laparoscopic specimen bag with subsequent removal as described. This does risk spillage and should be used with caution. Potential advantages to minimally invasive surgery include limiting the abdominal opening to several small incisions rather than the xiphoid to pubis incision often required for large mucinous neoplasms, with the concomitant decrease in hospital stay, earlier return to work, less pain, and less blood loss that characterize minimally invasive surgery [42]. Of course, such procedures require a skilled minimally invasive surgeon, and if intraperitoneal spill is deemed likely, the surgeon should immediately convert to a laparotomy.
Biologic Behavior
The biologic behavior of mucinous ovarian tumors depends on the specific histologic variant and stage. Intraepithelial (non-invasive) mOC, FIGO stage I, has a recurrence rate of only 5.8 % [36]. Patients with stage I invasive mOC have a 5-year survival rate of 91 %, whereas patients with advanced-stage tumor usually die of disease [16].
As a histologic group independent of stage, the prognosis for primary mOCs is better than that of its serous counterparts, due in large part to the high frequency of mucinous tumors being stage I at the time of diagnosis [22]. The average overall survival for over 6,000 women in the Swedish Family Center Database was just 34 months for patients with serous subtypes compared with 70 months for women with mucinous subtypes [43].
When considered stage for stage, however, women with advanced stage mOCs do significantly worse than women with other histologic subtypes of advanced stage ovarian cancer. In 2004, Hess et al., evaluated outcomes of stage III and IV patients with ovarian cancer who had undergone primary cytoreductive surgery followed by adjuvant therapy with a platinum agent. The matched cohort included 27 patients with mOC and 54 patients with non-mucinous ovarian cancer (2:1 match). Histologic grade, stage, optimal vs. suboptimal debulking status, chemotherapy regimen, and length of follow-up were no different between groups. However, the progression-free survival (PFS) for patients with mucinous ovarian cancer was 5.7 months, compared to 14.1 months for patients with non-mucinous ovarian cancer (p<0.001). Overall survival (OS) was also worse for patients with advanced stage mOC (12.0 months) compared with non-mucinous ovarian cancer (36.7 months) (p<0.001) [2].
These observations have been supported by Winter et al. [44], who reviewed the data from six phase III trials of adjuvant chemotherapy with cisplatin and paclitaxel conducted by the Gynecologic Oncology Group in women with stage III epithelial ovarian cancer after primary debulking surgery, both optimal and suboptimal. Only 2 % of the 1,895 patients had mOC, but their PFS was 10.5 months compared to 16.9 months for women with serous tumors, yielding a relative risk of progression of 2.18 for women with mOC (p<0.001). The difference in OS was also significant, as women with mOC had a median OS of 14.8 months compared to 45.2 months for women with serous ovarian cancer, yielding a relative risk of death from mucinous cancer of 4.14 (p<0.001).
The reason for such poor relative outcomes appears to be related to the frequency of platinum resistance in women with mucinous ovarian cancers. As noted, patients with early stage disease who do not require chemotherapy do well; conversely, patients with advanced stage disease who require chemotherapy in principle do relatively poorly [1]. Multiple authors have shown mOCs to be platinum resistant. Shimada et al. [9] found a lower response rate to platinum-based chemotherapy; the response rate among 24 women with mOC was 12.5 %, compared to 67.7 % among 189 women with serous ovarian carcinoma. Additionally, Pectasides et al. [45] performed a 1:2 matched study among women with advanced stage ovarian cancer who had received platinum-based chemotherapy within the Hellenic Cooperative Oncology Group. The response rate for 47 women with primary mOC was 38.5 % compared with 70 % for 94 women with serous histology. Survival differences were not significant.
Platinum resistance appears to extend to the recurrent setting. In a retrospective review of recurrent, platinum-sensitive epithelial ovarian carcinomas, patients with mucinous histology were less likely to respond to platinum-based regimens compared to patients with non-mucinous histology (36 % vs. 63 %, p=0.04). Similarly, PFS after recurrence was 4.5 months for patients with mucinous histology, compared to 8 months for patients with non-mucinous histology (p=0.03); OS was 17.9 months for the patients with mucinous carcinomas, compared to 28.8 months for patients with non-mucinous carcinomas (p=0.003) [46].
Pathologic Characteristics
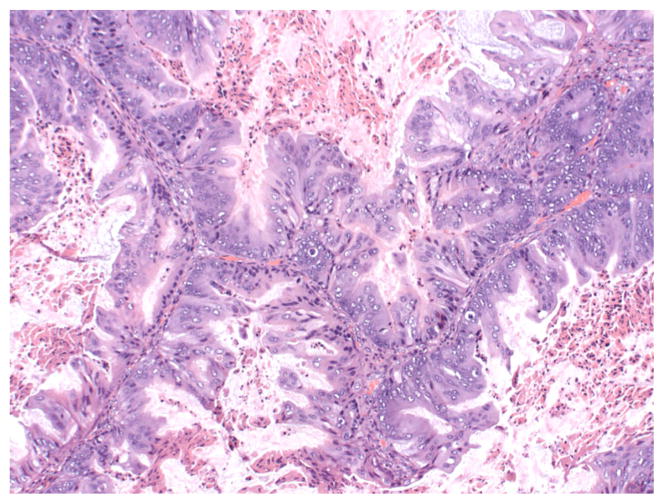
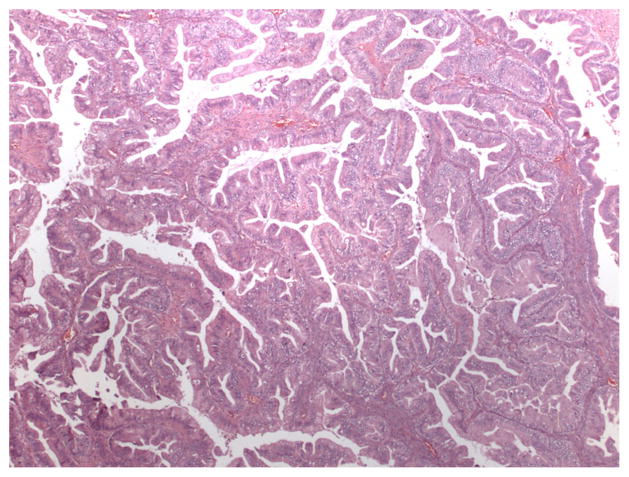
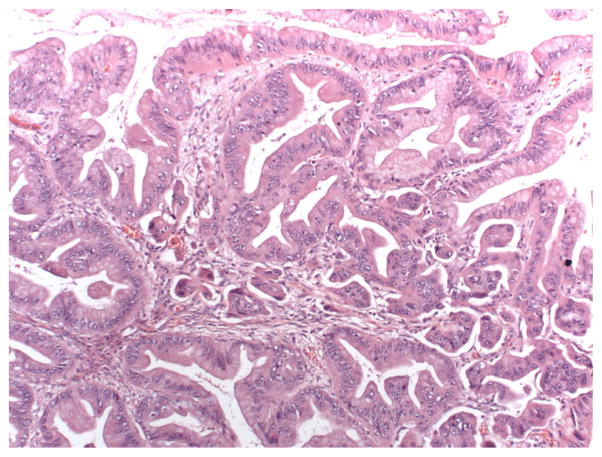
Ovarian mucinous neoplasms consist of borderline tumors (tumors of low malignant potential, or LMP tumors), intraepithelial (non-invasive) carcinoma, and invasive carcinoma. The World Health Organization criteria for the diagnosis of intestinal-type mucinous borderline tumor include the following elements: tumors contain cystic spaces lined by gastrointestinal-type mucinous epithelium with stratification and may form filiform papillae with at least minimal stromal support; nuclei are slightly larger than those seen in cystadenomas; mitotic activity is present; goblet cells and sometimes Paneth cells are present, but stromal invasion is absent [6]. Cases with marked cytologic atypia in the absence of stromal invasion represent intraepithelial carcinoma (see Fig. 1) [1]. The diagnosis of invasive mucinous carcinoma requires stromal invasion measuring more than 5 mm or more than 10 mm2 is detected. Invasive mucinous carcinoma is subdivided into expansile (confluent) type and infiltrative type [1]. The expansile, or confluent, type has a confluent glandular growth pattern without intervening normal ovarian parenchyma (see Fig. 2). The infiltrative pattern demonstrates small glands, nests or individual cells infiltrating the stroma, and tends to be more aggressive in clinical behavior than the expansile type (see Fig. 3) [36, 47].
Fig. 1.
10x H&E image showing intraepithelial carcinoma with stratified atypical nuclei with increased mitoses. Cytoplasm has decreased mucin. (Credit: Elizabeth Euscher, MD)
Fig. 2.
4x H&E image with expansile invasion on permanent section. (Credit: Elizabeth Euscher, MD)
Fig. 3.
10x H&E image with infiltrative invasion; small clusters of cells float in irregular cleft-like spaces. (Credit: Elizabeth Euscher, MD)
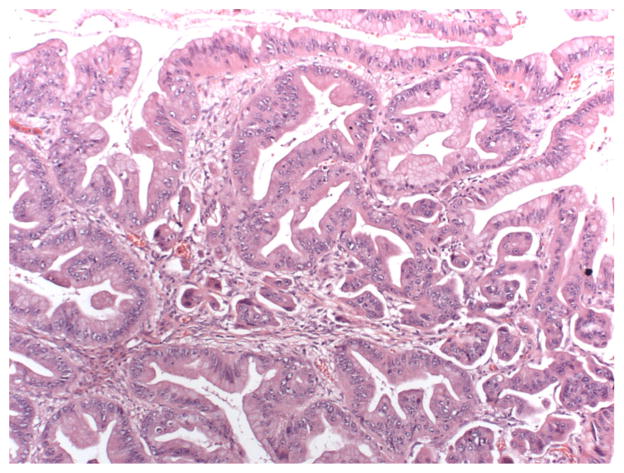
The distinction between these entities is subtle, and within one neoplasm, variations in degree of malignancy can be seen. Benign cystadenomas, LMP tumors, and invasive mucinous carcinomas can coexist in close proximity (see Fig. 4). This may suggest a continuum from benign to borderline to invasive disease. Practically, coexistence of these forms may make frozen section diagnosis difficult, as only a limited number of sections can be evaluated intraoperatively, and these tumors may be quite large [1, 6]. Additionally, this may contribute to inaccuracies in the diagnosis, as a small focus of invasive carcinoma may be overlooked amid a large neoplasm [6].
Fig. 4.
20x H&E image (same focus as the 10x); higher power view showing prominent nucleoli and coarse chromatin. Atypia similar to the intraepithelial carcinoma in the glands directly above the focus of invasion. (Credit: Elizabeth Euscher, MD)
The determination between primary mOC and mucinous tumors metastatic from other sites may be difficult. The most common primary sites for mucinous carcinomas metastatic to the ovary are gastrointestinal, pancreas, cervix, breast, and uterus [8]. Certain histological features can favor the diagnosis of primary mucinous carcinoma over metastatic disease, but discordant or overlapping features may prevent a definitive diagnosis. Primary mOC is favored by coexistent LMP and benign components, an expansile, or confluent, pattern of invasion, and other ovarian pathology, such as a mural nodule, Brenner tumor, or teratoma. Metastatic disease is supported by the following findings: a prominent desmoplastic response, nodular pattern of invasion, small clusters of tumor cells within corpora lutea or albicantia, numerous pools of mucin dissecting the ovarian stroma (i.e., pseudomyxoma ovarii) without a coexistent ovarian teratoma, extensive signet-ring cell pattern, ovarian surface involvement, vascular invasion, hilar involvement, and an extensive infiltrative pattern of invasion [14, 19].
Immunohistochemistry may also be useful in determining primary versus metastatic mOC. Primary mOCs often demonstrate CK7 and CK20 positivity, while colorectal primaries are usually positive only for CK20 [1]. Colorectal cancers also express racemase and β-catenin but primary mOCs do not. In situ hybridization for HPV may be useful in confirming an endocervical primary mucinous carcinoma metastatic to the ovary. Diffuse immunostaining of P16 may suggest endocervical origin, but this is only useful in cases of well differentiated adenocarcinoma, with the caveat that high grade ovarian mucinous or endometrioid adenocarcinomas can demonstrate p16 positivity [1]. Estrogen and progesterone receptors do not help distinguish between endocervical adenocarcinoma metastatic to the ovary and primary mucinous adenocarcinoma since both are usually progesterone receptor negative and estrogen receptor variable (either negative, weak and diffuse, or strong and focally positive). The latter have weak/diffuse or strong/focal staining [48]. Additionally, the presence of mesothelin, fascin, and prostate stem cell antigen (PSCA) favor a pancreatic primary metastatic to the ovary, while the presence of Dpc4 expression favors an ovarian primary [49]. When differentiating mucinous ovarian primary cancers from breast metastases, CK7 and CK20 will usually be positive for ovarian primaries, while only CK7 will be positive in metastatic breast malignancies; breast cancers will usually be positive for estrogen receptors and gross cystic disease fluid protein (GCDFP)-15, but mucinous ovarian primaries will not [50].
Molecular Signatures
Certain genetic mutations have been identified which characterize mOCs. KRAS mutations occur within the RAS family of G proteins, which signal cell division; such mutations stimulate cell growth and are significantly increased in mucinous ovarian tumors, including mucinous cystadenomas, LMP tumors, and invasive adenocarcinomas [51–53]. BRCA1 and BRCA2 mutations, genetic alterations in specific tumor suppressor genes that occur in many hereditary and some sporadic cases of breast and ovarian cancer, do not appear to be present in most cases of mOCs. Among patients with known BRCA mutations, only 2 % are of mucinous histology [54, 55]. Mutations in the p53 tumor suppressor gene are less frequent in mOCs than serous ovarian cancers; mutations in p53 have been found in almost 60 % of serous tumors but only 16 % of mucinous tumors [56]. Clear separation has also been seen in the gene expression profiling between serous and mOCs [57, 58].
Immunohistochemical studies have been used to characterize mucinous ovarian tumors. Mucinous tumors are more likely than serous tumors to express E-cadherin (62 % vs. 4 %, p<0.001) and less likely to stain positive for N-cadherin (8 % vs. 68 %, p<0.001) [59]. Other differences have been identified in the matrix metalloproteinases, p53, cadherin, CA125, and WT-1, among others [60–62]. Together, such molecular signatures suggest that mOCs are a separate entity with a pathogenesis different from other histologic subtypes of ovarian cancer.
Research Efforts
Since the pathogenesis and biologic behavior differ substantially from non-mucinous histologies of ovarian cancer, investigators worldwide have started to propose clinical trials that are specific to mOCs.
In the preclinical arena, multiple primary mOC cell lines have been treated with various cytotoxic chemotherapeutic agents, alone and in combination. As might be expected, all five cell lines were resistant to cisplatinum, carboplatin, and taxanes administered as single agents. Some cell lines showed sensitivity to oxaliplatin, etoposide, and 5-fluorouracil (5-FU) as single agents. In addition, the most active combination appeared to be oxaliplatin and 5-FU. This translated to improved outcomes in a mouse xenograft model [63].
Based on these data, this group of Japanese researchers is currently enrolling women with advanced or recurrent mOC in a single-arm phase II trial of S-1 and oxaliplatin. S-1 is an orally active drug made by Taiho Pharmaceuticals combining tegafur, a prodrug that is converted intracellularly to fluoururacil; gimeracil, a dihydropyrimidine dehydrogenase inhibitor; and oteracil, an inhibitor of fluorouracil phosphorylation, which reduces gastrointestinal toxicity [1].
An important next step is the international cooperative group trial currently being conducted by the GOG and the Gynecologic Cancer Intergroup. This is a 4-arm, phase III randomized study comparing carboplatin and paclitaxel with and without bevacizumab to oxaliplatin and capecitabine with and without bevacizumab in women with stages II–IV or recurrent, untreated stage I primary mucinous ovarian or fallopian tube cancer. The primary endpoint is overall survival; secondary endpoints include PFS, response rate, toxicity, and quality of life. Translational endpoints include KRAS mutations and expression of vascular endothelial growth factor and epidermal growth factor [1].
New directions include pursuing the role of Src kinase, a non-receptor tyrosine kinase, which regulates tumor progression through multiple signaling pathways. A novel targeted therapy exists which functions as a dual mechanism biologic agent, inhibiting Src kinase signaling and inhibiting pretubulin [62].
Conclusion
Mucinous ovarian tumors represent a distinct histologic entity. They differ significantly from other types of epithelial ovarian cancers in their pathogenesis, pathologic characteristics, molecular signature, and clinical behavior. These differences have established the need for innovative treatment approaches to achieve improved patient outcomes.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Jubilee Brown and Michael Frumovitz declare that they have no conflict of interest.
Contributor Information
Jubilee Brown, Email: jbbrown@mdanderson.org.
Michael Frumovitz, Email: mfrumovitz@mdanderson.org.
References
- 1.Frumovitz M, Schmeler KM, Malpica A, Sood AK, Gershenson DM. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol. 2010;117:491–6. doi: 10.1016/j.ygyno.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 3.Mondal SK, Banyopadhyay R, Nag DR, et al. Histologic pattern, bilaterality and clinical evaluation of 957 ovarian neoplasms: a 10-year study in a tertiary hospital in eastern India. J Cancer Res Ther. 2011;4:433–7. doi: 10.4103/0973-1482.92011. [DOI] [PubMed] [Google Scholar]
- 4.Koonings PP. Relative frequencies of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74:921–6. [PubMed] [Google Scholar]
- 5.Shappell HW, Riopel MA, Smith Sehdev AE, Ronnett BM, Kurman RJ. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002;26:1529–41. doi: 10.1097/00000478-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Chiesa AG, Deavers MT, Veras E, et al. Ovarian intestinal type mucinous borderline tumors: are we ready for a nomenclature change? Int J Gynecol Pathol. 2010;29:108–12. doi: 10.1097/PGP.0b013e3181bc2706. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinoma in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27:985–93. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Kigawa J, Ohishi Y, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113:331–4. doi: 10.1016/j.ygyno.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 11.Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin–paclitaxel versus cisplatin–cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 12.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106–15. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 13.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart WR, Norris HJ. Borderline and malignant mucinous tumours of the ovary: histologic criteria and clinical behavior. Cancer. 1973;31:1031–45. doi: 10.1002/1097-0142(197305)31:5<1031::aid-cncr2820310501>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Lack EE, Young RH, Scully RE. Pathology of ovarian neoplasms in childhood and adolescence. Pathol Annu. 1992;27:281–356. [PubMed] [Google Scholar]
- 16.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol. 1999;23:617–35. doi: 10.1097/00000478-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Tait DL, Miller DS. Multicystic ovarian tumor weighing 156 lbs: the second largest tumor in Texas. Tex Med. 1997;93:89. [PubMed] [Google Scholar]
- 18.Chao A, Chao A, Yen YS, Huang CH. Abdominal compartment syndrome secondary to ovarian mucinous cystadenoma. Obstet Gynecol. 2004;104:1180–2. doi: 10.1097/01.AOG.0000128106.96563.8b. [DOI] [PubMed] [Google Scholar]
- 19.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–92. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Boger-Megiddo I, Weiss NS. Histologic subtypes and laterality of primary epithelial ovarian tumors. Gynecol Oncol. 2005;97:80–3. doi: 10.1016/j.ygyno.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Khunamornpong S, Suprasert P, Pojchamarnwiputh S, et al. Primary and metastatic mucinous adenocarcinomas of the ovary: evaluation of the diagnostic approach using tumor size and laterality. Gynecol Oncol. 2006;101:152–7. doi: 10.1016/j.ygyno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Seidman JD, Horkayne-Szakaly I, Haiba M, et al. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–4. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 23.Cho YH, Kim DY, Kim JH, et al. Is complete surgical staging necessary in patients with stage I mucinous epithelial ovarian tumors? Gynecol Oncol. 2006;103:878–82. doi: 10.1016/j.ygyno.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Roger N, Zafrani Y, Uzan C, et al. Should pelvic and para-aortic lymphadenectomy be different depending on histological subtype in epithelial ovarian cancer? Ann Surg Oncol. 2008;15:333–8. doi: 10.1245/s10434-007-9639-6. [DOI] [PubMed] [Google Scholar]
- 25.Schmeler KM, Tao X, Frumovitz M, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol. 2010;116:269–73. doi: 10.1097/AOG.0b013e3181e7961d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leblanc E, Querleu D, Narducci F, et al. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–9. doi: 10.1016/j.ygyno.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 27.Tholander B, Taube A, Lindgren A, et al. Pretreatment serum levels of CA-125, carcinoembryonic antigen, tissue polypeptide antigen, and placental alkaline phosphatase in patients with ovarian carcinoma: influence of histological type, grade of differentiation, and clinical stage of disease. Gynecol Oncol. 1990;39:26–33. doi: 10.1016/0090-8258(90)90394-z. [DOI] [PubMed] [Google Scholar]
- 28.Tuxen MK, Soletormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995;21:215–45. doi: 10.1016/0305-7372(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 29.Nolen B, Marrangoni A, Velikokhatnaya L, et al. A serum based analysis of ovarian epithelial tumorigenesis. Gynecol Oncol. 2009;112:47–54. doi: 10.1016/j.ygyno.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The effect of maximal surgical cytoreduction on sensitivity to platinum-taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecol Oncol. 2008;108:276–81. doi: 10.1016/j.ygyno.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Fleming GF, Ronnett BM, Seidman J, et al. Epithelial ovarian cancer. In: Barakat RR, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 763–835. [Google Scholar]
- 32. [accessed on 10/1/2013];NCCN Guidelines - Ovarian Cancer, Version 2.2013. [Google Scholar]
- 33.Schilder JM, Thompson AM, DePriest PD, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 34.Wright JD, Shah M, Mathew L, et al. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115:4118–26. doi: 10.1002/cncr.24461. [DOI] [PubMed] [Google Scholar]
- 35.Gershenson DM. Treatment of ovarian cancer in young women. Clin Obstet Gynecol. 2012;55:65–74. doi: 10.1097/GRF.0b013e318248045b. [DOI] [PubMed] [Google Scholar]
- 36.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘Pseudomyxoma Peritonei’. Am J Surg Pathol. 2000;24:1447–64. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Lin JE, Seo S, Kushner DM, Rose SL. The role of appendectomy for mucinous ovarian neoplasms. Am J Obstet Gynecol. 2013;208:46, e1–4. doi: 10.1016/j.ajog.2012.10.863. [DOI] [PubMed] [Google Scholar]
- 38.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 39.Naik JD, Seligman J, Perren TJ. Mucinous tumors of the ovary. J Clin Pathol. 2012;65:580–4. doi: 10.1136/jclinpath-2011-200320. [DOI] [PubMed] [Google Scholar]
- 40.Link CJ, Jr, Reed E, Sarosy G, et al. Borderline ovarian tumors. Am J Med. 1996;101:217–25. doi: 10.1016/s0002-9343(96)80079-9. [DOI] [PubMed] [Google Scholar]
- 41.Leys CM, Gasior AC, Hornberger LH, St Peter SD. Laparoscopic resection of massive ovarian mucinous cystadenoma. J Laparoendosc Adv Surg Tech. 2012;22:307–10. doi: 10.1089/lap.2011.0435. [DOI] [PubMed] [Google Scholar]
- 42.Eltabbakh GH, Charbonneau AM, Eltabbakh NG. Laparoscopic surgery for large benign ovarian cysts. Gynecol Oncol. 2008;108:72–6. doi: 10.1016/j.ygyno.2007.08.085. [DOI] [PubMed] [Google Scholar]
- 43.Ji J, Forsti A, Sundquist J, Lenner P, Hemminki K. Survival in ovarian cancer patients by histology and family history. Acta Oncol. 2008;47:1133–9. doi: 10.1080/02841860701784544. [DOI] [PubMed] [Google Scholar]
- 44.Winter WE, III, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 45.Pectasides D, Fountzilas G, Aravantinos G, et al. Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol. 2005;97:436–41. doi: 10.1016/j.ygyno.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 46.Pignata S, Ferrandina G, Scarfone G, Scollo P, Odicino F, Cormio G, et al. Activity of chemotherapy in mucinous ovarian cancer with a recurrence free interval of more than 6 months: results from the SOCRATES retrospective study. BMC Cancer. 2008;8:252. doi: 10.1186/1471-2407-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–52. doi: 10.1097/00000478-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Ronnett BM, Yemelyanova AV, Vang R, et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumors and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32:1835–53. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- 49.Cao D, Ji H, Ronnett BM. Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int J Gynecol Pathol. 2005;24:67–72. [PubMed] [Google Scholar]
- 50.Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36:8–37. doi: 10.1053/j.seminoncol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 52.Gemignani ML, Schlaerth AC, Bogomolniy F, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–81. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 53.Mok SC, Bell DA, Knapp RC, et al. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 54.Tonin PN, Maugard CM, Perret C, Mes-Masson AM, Provencher DM. A review of histopathological subtypes of ovarian cancer in BRCA-related French Canadian cancer families. Fam Cancer. 2007;6:491–7. doi: 10.1007/s10689-007-9152-x. [DOI] [PubMed] [Google Scholar]
- 55.Evans DG, Young K, Bulman M, et al. Probability of BRCA1/2 mutation varies with ovarian histology: results from screening 442 ovarian cancer families. Clin Genet. 2008;73:338–45. doi: 10.1111/j.1399-0004.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 56.Schuijer M, Berns EM. TP53 and ovarian cancer. Hum Mutat. 2003;21:285–91. doi: 10.1002/humu.10181. [DOI] [PubMed] [Google Scholar]
- 57.Marchini S, Mariani P, Chiorino G, et al. Analysis of gene expression in early-stage ovarian cancer. Clin Cancer Res. 2008;14:7850–60. doi: 10.1158/1078-0432.CCR-08-0523. [DOI] [PubMed] [Google Scholar]
- 58.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–13. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarrio D, Moreno-Bueno G, Sanchez-Estevez C, et al. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum Pathol. 2006;37:1042–9. doi: 10.1016/j.humpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Brun JL, Cortez A, Commo F, Uzan S, Rouzier R, Darai E. Serous and mucinous ovarian tumors express different profiles of MMP-2, -7, -9, MT1-MMP, and TIMP-1 and -2. Int J Oncol. 2008;33:1239–46. [PubMed] [Google Scholar]
- 61.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuo K, Masato M, Nishimura M, et al. Targeting Src in mucinous ovarian carcinoma. Clin Cancer Res. 2011;17:5367–78. doi: 10.1158/1078-0432.CCR-10-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato S, Itamochi H, Kigawa J, et al. Combination chemotherapy of oxaliplatin and 5-fluorouracil may be an effective regimen for mucinous adenocarcinoma of the ovary: a potential treatment strategy. Cancer Sci. 2009;100:546–51. doi: 10.1111/j.1349-7006.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]