Abstract
The optimal pretransplant regimen for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in patients ≥55 years of age remains to be determined. The myeloablative reduced-toxicity 4-day regimen IV busulfan (Bu) (130 mg/m2)-IV fludarabine (Flu) (40 mg/m2) is associated with low morbidity and mortality. We analyzed 79 patients ≥55 years of age (median, 58 years) with AML (n=63) or MDS (n=16) treated with IV Bu-Flu conditioning regimens between 2001 and 2009 (median follow-up, 24 months). The patients who received this regimen had a good performance status. The 2-year overall survival rates for patients in first complete remission (CR1), second CR (CR2), refractory disease and for all patients at time of transplantation were 71%, 44%, 32%, and 46%, respectively; 2-year event-free survival rates for patients in CR1, CR2, or refractory disease at time of transplantation and for all patients were 68%, 42%, 30%, and 44%, respectively. One-year transplant-related mortality (TRM) rates for patients who were in CR or who had active disease at the time of transplantation were 19% and 20%, respectively. Grade II-IV acute graft-versus-host disease was diagnosed in 40% of the patients. Our results suggest that age alone should not be the primary reason for exclusion from receiving myeloablative reduced-toxicity conditioning with IV Bu-Flu preceding transplantation in patients with AML/MDS.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly, with a median patient age at diagnosis of 70 years (1). AML is an aggressive disease with AML patients having a 5-year relative survival rate of 5% to 15%. Patient-, disease-, and donor-related issues limit and, at times, exclude the use of allogeneic hematopoietic stem cell transplantation (allo-SCT), which can be curative. Many factors contribute to the adverse outcomes of allo-SCT in elderly patients with AML (2-4). First, age-associated comorbid conditions, impact the tolerance to chemotherapy. Second, many pharmaceutically active agents are metabolized differently in older individuals (5, 6) and total systemic exposure of the conditioning therapy is not only related to overall toxicity, but also to acute graft-versus-host disease (GvHD), presumably through cytokine release from damaged organs (7). Third, the incidence of GvHD increases with age; this higher incidence of GvHD is partly due to an altered immune response, such as enhanced allogeneic stimulatory activities of antigen-presenting cells (8). Fourth, the potential of T-cells to mediate antileukemia activities is reduced in elderly patients, thereby limiting the beneficial graft-versus-leukemia effect. Additionally, the diversity in the naive T-cell repertoire is decreased after the seventh decade of life; this deficiency is compounded by a reduced ability of the thymus to rebuild a broad T-cell repertoire to target a wide range of antigens (9). Finally, stem cell homing, and therefore engraftment, may also be adversely affected by aging (10). These deficiencies underscore the critical need for a regimen that will maximize the eradication of residual leukemia cells and allow prompt engraftment with rapid reconstitution of immune response to mediate optimal antileukemia immunity.
Effective cytoreduction is critical in the treatment of elderly patients with AML, as it reduces the disease burden, permits rapid engraftment and immune reconstitution with long-term disease-control. If an ablative regimen can be safely delivered to patients up to and beyond 65 years of age, the currently held notion that reduced-intensity conditioning (RIC) is the optimal approach in this age group would be challenged. Paradoxically, from the antileukemia standpoint, and also in consideration of the immunological challenges imposed by aging, an optimally cytoreductive, myeloablative conditioning regimen may be even more desirable in older patients.
The reduced-toxicity IV busulfan (Bu)-fludarabine (Flu) regimen was shown to be a safe myeloablative conditioning program when compared with the more commonly used regimen of Bu-cyclophosphamide (Cy) in the treatment of patients with AML (11). In that study the outcome of 67 patients who received the Bu-Cy2 regimen were compared with 148 patients who received the Bu-Flu conditioning regimen prior to allo-SCT for AML; patients whose disease was in first complete remission (CR1) who received Bu-Flu had significantly better 3-year overall survival (OS) and event-free survival (EFS) rates than those who received Bu-Cy, despite the higher median age of the Bu-Flu population (46 versus 39 years) (11). Of importance, the 3-year treatment-related mortality, which is often attributed to the conditioning regimen’s toxicities, was significantly lower in the Bu-Flu group (14% versus 34%). In our early study (11), we had transplanted some patients who were in the 7th decade of life using the Bu-Flu regimen, and our impression was that the results would support the use of IV Bu-Flu in “elderly patients”-- in this report defined as being older than 50-55 years. Based on the success of Bu-Flu in this age bracket, we hypothesized that IV Bu-Flu might be a safe and effective conditioning therapy in elderly patients prior to allo-SCT for AML and MDS.
In view of this hypothesis, we retrospective analyzed 79 patients treated with the reduced-toxicity myeloablative regimen IV Bu-Flu. We critically examined the use of IV Bu-Flu followed by allo-SCT for AML and MDS patients in the 6th to 8th decades of life, and the present manuscript describes details of this investigation. Our data support the systematic use of the IV Bu-Flu conditioning regimen in high-risk patients older than 50-55 years, if they have a good performance status.
Materials and methods
Patients
We studied all patients 55 years or older who had been treated in 2 consecutive prospective protocols conducted at our institution (The University of Texas MD Anderson Cancer Center) and 5 patients treated with the same regimen in a prospective clinical trial at Hospital Israelita Albert Einstein (Sao Paulo, Brazil). The first protocol was a phase II study that investigated high-dose IV Bu-Flu with fixed dose delivery (12), while the second protocol compared a regimen of IV Bu-Flu with fixed-dose Bu with one containing dose-adjusted Bu in which pharmacokinetic monitoring targeted a systemic exposure of Bu as represented by the average daily area under the concentration vs. time curve for Bu of 6000 μMol*min for each of the 4 days of the regimen, similar to previous reports(13, 14). The studies were approved by institutional review board and all patients provided written informed consent as per institutional guidelines. IV busulfan was purchased from Otsuka America Pharmaceutical, Inc. (Princeton, NJ, USA).
To be eligible for these studies, patients were required to have adequate normal organ functions: hepatic function (SGPT ≤ 200 IU/mL, serum bilirubin and alkaline phosphatase within accepted laboratory standard normal limits or considered not clinically significant, and no evidence of chronic active hepatitis or cirrhosis); renal function (serum creatinine ≤ 1.5 mg/dL); cardiac function (left ventricular ejection fraction >45%; no uncontrolled arrhythmias or symptomatic cardiac disease); and pulmonary function (no symptomatic pulmonary disease, and FEV1, FVC and DLCO ≥ 50% of expected corrected for hemoglobin). AML patients were required to have disease status past first remission, primary induction failure, in first or subsequent relapse, or in first remission with intermediate or high-risk cytogenetics; MDS with intermediate or high risk International Prognostic Scoring System score; no systemic therapy administered within 21 days prior to trial enrollment; no active infection; and ZUBROD performance status <2. Good-risk cytogenetics included patients with translocation (t)(8;21); inversion (inv)(16) or t(16;16); deletion (del)(9q); or t(15;17). Intermediate-risk cytogenetics included patient with a normal karyotype; -Y; del (5q); loss of 7q; t(9;11); +11; del(11q); abnormality (12p); +13; del(20q); or +21. High-risk cytogenetics included patients with a complex karyotype (≥3 abnormalities); inv(3) or t(3;3); t(6;9); t(6;11); -7; +8 (sole abnormality); or +8 with one other abnormality other than t(8;21), t(9;11), inv(16), or t(16;16); t(11;19)(q23;p13.1) (15).
The conditioning regimen consisted of IV Flu (40 mg/m2) (Genzyme Inc., Cambridge, MA, USA) and IV Bu (130 mg/m2) administered over a 3-hour period once a day on pretransplant days -6 to -3 (12). Tacrolimus and mini-methotrexate were used for GvHD prophylaxis (16), and pentostatin (Hospira Inc., Lake Forest, IL, USA) was administered in 13 cases (18%). Thymoglobulin (4 mg/kg) (Genzyme Inc., Cambridge, MA, USA) was administered to those who received grafts from mismatched related or unrelated donors on days -3 to -1. All donor-recipient pairs were fully typed at high resolution for the alleles of HLA-A, -B, -C, -DR, -DQ.
Statistical analysis
The primary outcomes of interest in the current analysis were OS and EFS rates, cumulative incidence of transplant-related mortality (TRM), and cumulative incidence of acute GvHD. All outcomes were evaluated from the date of transplantation, with the exception of monitoring for regimen-related adverse events, which included the days of chemotherapy administration. The probabilities of survival were estimated using the Kaplan-Meier method (17, 18). The log-rank test was used to compare survival probabilities between subgroups of patients. The cumulative incidence of TRM was estimated by considering death due to disease relapse and/or resistance or any other non-treatment-related cause as a competing risk. The cumulative incidence of acute GvHD was estimated by considering disease progression or death due to any cause as a competing risk. All statistical analyses were performed using SAS and S-plus software (SAS Institute Inc., Cary, NC). P values less than 0.05 were deemed statistically significant (17, 18).
Results
Patients
Seventy-nine patients 55 years or older with either AML (n = 63; secondary AML [n = 8]) or MDS (n = 16) underwent allo-SCT from September 2001 to May 2009. Pretransplant patient characteristics are presented in Table 1. The median age at the time of transplantation was 58 years (range, 55-76 years); 5 patients were older than 65. The median survival was 8 months (range, 1-82 months). Eighty percent (n=20) and 16% (n=4) of patients in CR1 had intermediate- and poor-risk cytogenetics, respectively, and cytogenetics were unknown in 1 patient. Six percent (n=1), 82% (n=14) and 6% (n=1) of patients in CR2 had good-, intermediate-, and poor-risk cytogenetics, respectively; cytogenetics were unknown in 1 patient. The median hematopoietic stem cell transplantation comorbidity index (HCT-CI) score was 1 (19).
Table 1.
Pretransplant patient characteristics
| Characteristic | Number (%) | |
|---|---|---|
| Gender | Male | 49 (62) |
| Female | 30 (38) | |
| Age | >58 | 40 (51) |
| ≤58 | 39 (49) | |
| Disease | AML | 63 (80) |
| MDS | 16 (20) | |
| Disease status at time of allo-SCT | CR | 42 (53) |
| CR1 | 25 (32) | |
| CR2 | 17 (22) | |
| Active disease | 37 (47) | |
| Cytogenetics | Good risk | 4 (5) |
| Intermediate risk | 50 (63) | |
| Poor risk | 20 (25) | |
| Unknown | 5 (6) | |
| Graft received | Related Donor | 41 (52) |
| Unrelated Donor | 38 (48) | |
| Stem cell source | Bone marrow | 38 (48) |
| Peripheral blood | 41 (52) | |
| HCT-CI Score* | ≤ 1 | 41 (52) |
| 2 | 9 (11) | |
| ≥3 | 27 (34) |
Percentages listed are of the total number of patients (n=79).
Data were unavailable for 2 patients.
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; allo-SCT, allogeneic stem cell transplantation; CR, complete remission; CR1, first complete remission; CR2, second complete remission; HCT-CI, hematopoietic stem cell transplantation comorbidity index(19).
Overall and event-free survival
The 2-year OS and EFS rates for the 25 patients who underwent transplantation while in CR1 were 71% and 68% respectively (Table 2; Figure 1 A-B). The 2-year OS and EFS rates for the entire cohort were 46% and 44%, respectively (Figure 1C-D). The 17 patients who underwent transplantation while their disease was in second complete remission (CR2) had 44% 2-year OS rate while the 37 patients who had active disease at the time of allo-SCT had 32% 2-year OS rate. Thirty-two percent of patients had disease progression (n=25). Disease status was the strongest predictor of OS time (P=0.006), with patients in CR1 having the longest OS time, followed by those in CR2 and those with active disease at time of transplantation.
Table 2.
Post-transplant Outcomes
| Characteristic | Number (%) | |
|---|---|---|
|
| ||
| GvHD | Acute, Grade II-IV | 32 (41) |
| Acute, Grade III-IV | 5 (7) | |
| Chronic | 34 (43) | |
|
| ||
| Cause of death* | Disease progression | 23 (58) |
| GvHD | 9 (23) | |
| Infection | 4 (10) | |
| Organ failure | 3 (8) | |
|
| ||
| Kaplan-Meier estimate (95% CI) | ||
|
| ||
| 2-year OS† | CR1 | 71% (54% -94%) |
| CR2 | 44% (24% -81%) | |
| Active disease | 32% (19% -53%) | |
|
| ||
| 2-year EFS† | CR1 | 68% (51% -91%) |
| CR2 | 42% (23% -77%) | |
| Active disease | 30% (18% -50%) | |
Except as noted, all percentages are calculated for the entire cohort (n = 79).
Percentages reported in each cause of death category were calculated from the total number of patients who died (n = 40).
Percentages reported for 2-year OS and EFS were categorized by disease status at the time of allo-SCT.
GvHD indicates graft-versus-host disease; OS, overall survival; CR1, first complete remission; CR2, second complete remission; and EFS, event-free survival.
Figure 1. Kaplan-Meier curves for OS and EFS.
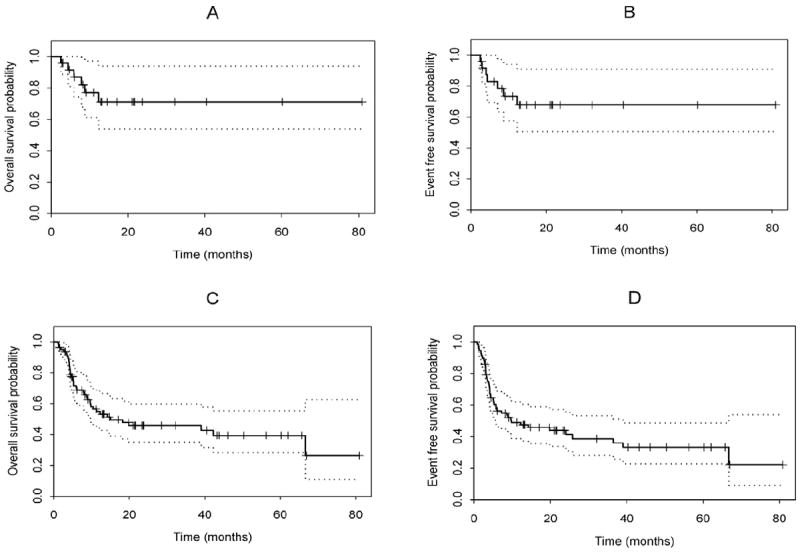
OS and EFS probabilities for (A-B) patients in CR1 (n=25) and (C-D) for entire cohort (n=79).
Post-transplant outcomes, graft-versus-host disease and transplant-related mortality
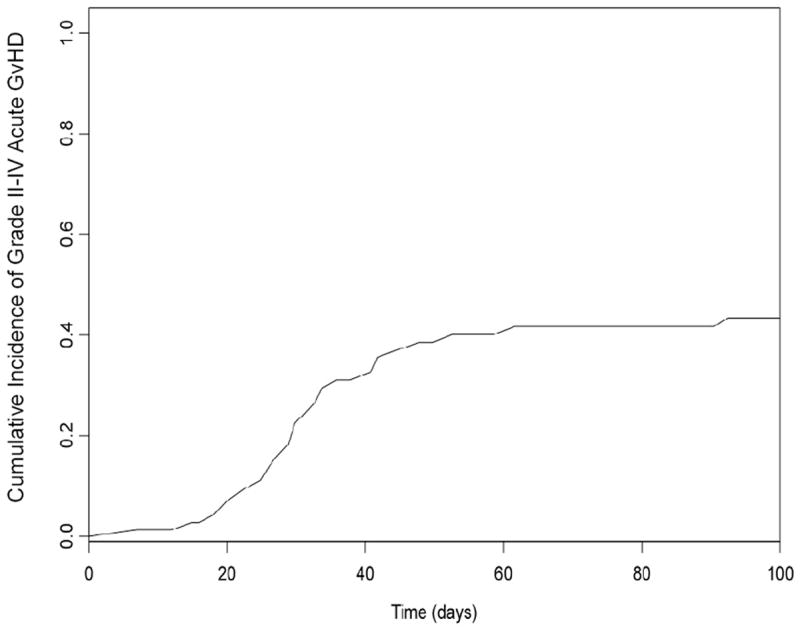
Post-transplant outcomes are reported in Table 2. Engraftment was achieved in all patients. At day +30, 73% of patients (n=56) had achieved full donor chimerism and 23% (n=18) mixed chimerism (defined as the presence of any recipient cell or DNA as detected by cytogenetics, fluorescence in situ hybridization or DNA microsatellite polymorphisms); chimerism data was unavailable for 5 patients. Overall 100-day mortality was 6% (n=5) and was due to secondary graft failure (n=1), persistence of disease (n=1), liver failure (n=1), CNS bleed (n=1) and pre-existing radiation induced brain toxicity (n=1). Forty one percent of patients (n=32) developed grade II-IV acute GvHD, 7% (n=5) developed grade III-IV acute GvHD, and 43% (n=34) developed chronic GvHD. The cumulative incidence of acute GvHD (grades II-IV) is shown in Figure 2. Our analysis also showed that the HCT-CI score was higher (median score, 2) in patients with grade II-IV acute GvHD, compared with patients who had acute GvHD grades 0-1 (median score, 1) (P=0.02).
Figure 2. Cumulative incidence of grade II-IV acute graft-versus-host disease (GvHD).
TRM was 1% at day +30 for all patients, and was highest in patients who underwent stem cell transplantation with active disease. TRM rates at day +100 for patients who underwent stem cell transplantation while in CR and for patients with active disease at time of stem cell transplantation were 5% for both groups. One-year TRM rates for patients who were in CR or who had active disease at the time of stem cell transplantation were 19% and 20%, respectively (Table 3).
Table 3.
Transplant-related Mortality.
| Transplant-related mortality | 30 days | 100 days | 1 year | 3 year |
|---|---|---|---|---|
| All patients (n=79) | 1% | 5% | 19% | 26% |
| All CR at allo-SCT (n = 42) | None | 5% | 19 % | 26% |
| CR1 at allo-SCT (n = 25) | None | 4% | 14% | 19% |
| Active disease (n = 37) | 3% | 5% | 20% | 27% |
Percentages shown are calculated based on the total number of patients in each category.
CR indicates complete remission; allo-SCT, allogeneic stem cell transplantation; and CR1, first complete remission.
Effects of age on overall survival
We further investigated whether age at the time of allo-SCT affected OS rates. For this analysis, we allocated the patients into 2 groups using the median age of 58 years as the cutoff (i.e.< 58 versus ≥ 58 years). Our results showed that OS was not significantly affected by age (median follow-up, 24 months) (Figure 3). Our data also showed that age alone did not affect outcome, regardless of disease status at the time of transplantation (Figure 4).
Figure 3. Overall survival in patients 58 years or older versus patients younger than 58 years.
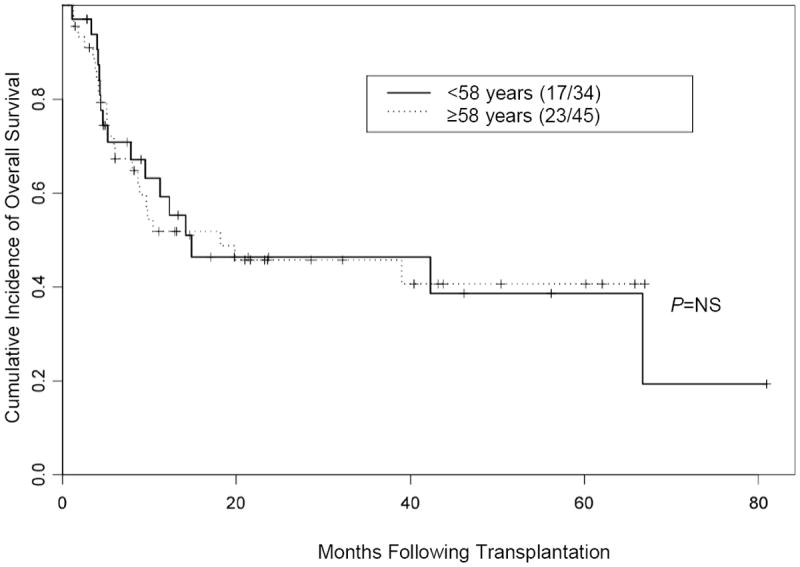
NS indicates not significant.
Figure 4. Kaplan-Meier curve for overall survival according to remission status at time of transplantation.
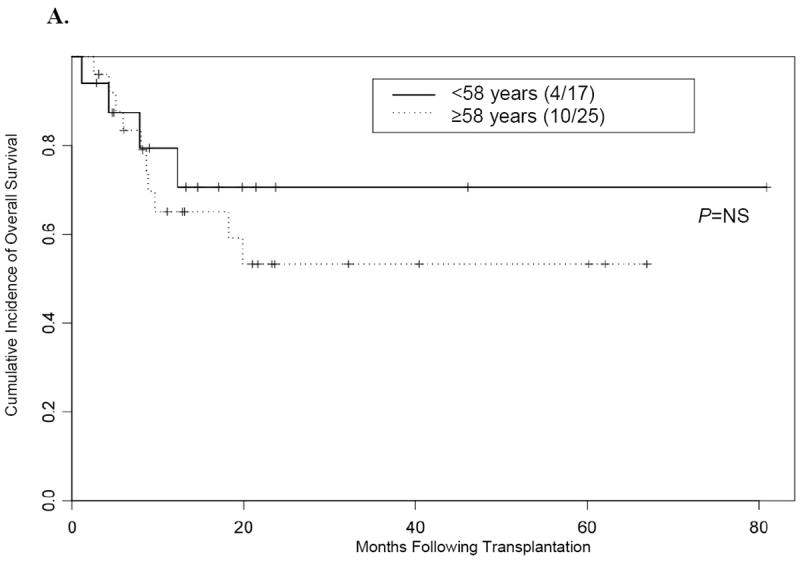
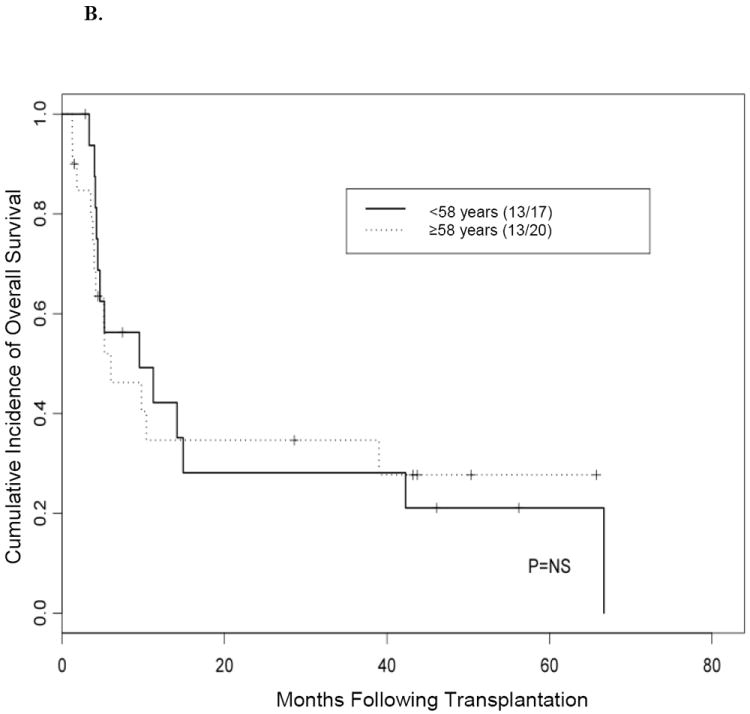
(A) Overall survival in patients 58 years or older versus patients younger than 58 years who underwent transplantation while their disease was in complete remission. (B) Overall survival in patients 58 years or older versus patients younger than 58 years who underwent transplantation while they had active disease.
NS indicates not significant.
Discussion
In this analysis of 79 patients with AML or MDS who were ≥ 55 years of age at time of allo-SCT, we showed good outcomes including OS, EFS, TRM and GvHD rates following the use of the myeloablative, reduced-toxicity IV Bu-Flu regimen. Since it remains unknown if there should be an optimal age to use for excluding patients from allo-SCT or from receiving this type of myeloablative regimens, and since many older adults with AML or MDS are offered RIC regimens prior to allo-SCT purely because of their age, our results challenge the current treatment tradition of offering mostly reduced-intensity programs for older patients with AML and MDS. Furthermore, our findings are especially important since AML and MDS are of intermediate sensitivity to the graft-versus-leukemia effect, and higher doses of chemotherapeutic agents are often required for effective disease control (20, 21). Therefore, we show that selected patients ≥55 years of age with AML or MDS can be safely treated with the reduced-toxicity IV Bu-Flu regimen, with acceptable overall outcomes and encouraging long-term disease control.
Our results are consistent with our prior report that employed IV Bu-Flu as a conditioning regimen for allo-SCT in a cohort of patients with a median age of 46 years (11, 12). The 2-year OS and EFS rates we observed here for patients whose disease was in CR1 were 71% and 68%, respectively; these rates were similar to the OS and EFS rates (78% and 74%, respectively) that we previously reported using this myeloablative IV Bu-Flu regimen (11, 12). Additionally, our outcomes compare favorably to RIC IV Bu-Flu (22, 23), which in older (≥60 years, median age, 63 years) leukemia patients (62% with AML or MDS) was shown to have 2-year cumulative incidence of non-relapse mortality NRM and OS of 10% and 46%, respectively (22).
It has lately become a practice to exclude older AML or MDS patients from myeloablative conditioning regimens. This is unfortunate, knowing that, in comparison with RIC regimens, a more potent disease eradication may yield a distinct benefit in patients. In one study, elderly patients (median age, 64 years) treated with the RIC regimens consisting of Flu, melphalan, and carmustine followed by allo-SCT achieved 1-year OS, disease-free survival, and NRM rates of 68%, 61%, and 22%, respectively (24). Another study in older patients (median age, 53 years) using a non-myeloablative conditioning regimen with IV Flu and oral Bu showed 4-year OS and EFS and 1-year NRM rates of 42%, 44%, and 16%, respectively (25). We have previously reported 3-year OS and EFS and 1-year NRM rates of approximately 40%, 20%, and 20%, respectively, in older patients (average age, 61 years) who received the non-myeloablative regimen Flu, cytarabine, and idarubicin (20).
Previous studies using myeloablative conditioning regimens prior to allo-SCT in elderly patients have reported poor outcomes secondary to high NRM rates. Wallen et al (26) evaluated ablative allo-SCT in adults 60 years or older (median age, 63 years) in which most patients received Bu-Cy (67%) or total-body irradiation (TBI)-Cy (21%), and only 10% of the patients received Bu-Flu; 3-year OS and relapse rates were estimated to be 34% and 24%, respectively; 100-day and 3-year NRM rates were 27% (versus 5% in our study) and 43%, respectively; and the acute GvHD (grade III-IV) rate was 20% (versus 7% in our study). In a subgroup analysis of older patients with AML who received unrelated donor grafts preceded by RIC or myeloablative conditioning regimens, Ringden et al (27) showed that patients 50 years or older (median age, 54 years) who received myeloablative conditioning regimens had 2-year leukemia-free survival, NRM, and acute GvHD (grade II-IV) rates of 43%, 39%, and 29%, respectively. However, similar to the study by Wallen et al (26), in the report by Ringden et al (27) only 14% of patients 50 years or older received Bu-Flu, whereas the majority of patients received either TBI-Cy (47%) or Bu-Cy (34%).
Cahn et al (28) retrospectively compared allo-SCT in AML patients over 40 years old to those age 40 and younger who underwent transplantation while their disease was in CR1 and reported similar relapse incidence and OS rates between the 2 groups; however, the rate of TRM was higher in patients older than 40 years, and most of these patients received Cy-TBI or Bu-Cy as their conditioning regimens. Likewise, in an analysis of 71 patients with de novo MDS who received Cy-TBI or Bu-Cy conditioning regimens, Sutton et al (29) reported shorter OS and EFS times and a higher relapse rate among patients who underwent transplantation at a later age (median, 37 years). We recognize the plethora of data that support RIC regimens in older AML and MDS patients, but when contrasted with these previous studies, our results highlight the need to re-evaluate age as an exclusion criterion for myeloablative regimens in light of the improved outcomes with IV Bu-Flu.
Recently, studies of patients 50 years or older with AML or MDS were conducted by the European Group for Blood and Marrow Transplantation and showed increased disease relapse rates following RIC compared with myeloablative conditioning regimens; in essence, the increase in disease relapse for RIC offsets the increase in TRM for the myeloablative regimens (27). Our analysis does not compare current data with historical controls, and in the absence of randomized trials, the issue of dose intensity remains unresolved. However, our findings suggest that older patients can benefit from the reduced-toxicity, myeloablative IV Bu-Flu regimen, which we showed likely to be less toxic and significantly safer than other commonly used myeloablative regimens. It is assumed that providing optimized cytoreduction contributes to improved long-term disease control (20, 30).
In view of our results, we recommend that the reduced-toxicity IV Bu-Flu regimen be considered for patients up to at least age 65, unless they suffer serious comorbid conditions. The tolerance for this therapy in elderly patients with serious comorbidities has not been evaluated under stringent, well-controlled conditions, and such patients may benefit from additional precautions. The regimen could however be considered for selected patients with comorbid conditions in this age group, if the treatment is given in a controlled clinical study to evaluate the tolerance to administered treatment in such a setting. As previously demonstrated in younger patients, the regimen seems especially fit for patients whose disease is in CR (11, 12). For patients whose disease is not in CR at the time of allo-SCT, to date, no regimen has been identified as the “standard conditioning regimen” and a variety of approaches are under investigation, including the replacement or supplementation of fludarabine with clofarabine (31) complementation of IV Bu-Flu with low-dose TBI (32) and/or post-allo-SCT maintenance therapy to prolong CR in high-risk patients (33).
Acknowledgments
Salary for Dr. Alatrash at the time this work was conducted was supported in part by the MD Anderson Barbara Rattay Advanced Scholars Program.
These studies were supported by NIH grants CA55164 and CA49639
Footnotes
Financial disclosure: B. S. Andersson is a consultant to Otsuka America Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.de Lima M, Champlin R. Unrelated donor hematopoietic transplantation. Rev Clin Exp Hematol. 2001;5:100–134. doi: 10.1046/j.1468-0734.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 3.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 4.McGlave PB, Shu XO, Wen W, et al. Unrelated donor marrow transplantation for chronic myelogenous leukemia: 9 years’ experience of the national marrow donor program. Blood. 2000;95:2219–2225. [PubMed] [Google Scholar]
- 5.Fleming RA, Capizzi RL, Rosner GL, et al. Clinical pharmacology of cytarabine in patients with acute myeloid leukemia: a cancer and leukemia group B study. Cancer Chemother Pharmacol. 1995;36:425–430. doi: 10.1007/BF00686192. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JL. Pharmacokinetic changes in aging. Am J Med. 1986;80:31–38. doi: 10.1016/0002-9343(86)90535-8. [DOI] [PubMed] [Google Scholar]
- 7.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 8.Ordemann R, Hutchinson R, Friedman J, et al. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 13.Geddes M, Kangarloo SB, Naveed F, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Khouri I, Ippoliti C, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant. 1999;24:763–768. doi: 10.1038/sj.bmt.1701983. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–481. [Google Scholar]
- 18.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 19.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 20.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 21.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 22.Koreth J, Aldridge J, Kim HT, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant. 2010;16:792–800. doi: 10.1016/j.bbmt.2009.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohty M, de Lavallade H, El-Cheikh J, et al. Reduced intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia: long term results of a ‘donor’ versus ‘no donor’ comparison. Leukemia. 2009;23:194–196. doi: 10.1038/leu.2008.164. [DOI] [PubMed] [Google Scholar]
- 24.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21:1480–1484. doi: 10.1200/JCO.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 25.Valcarcel D, Martino R, Caballero D, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 26.Wallen H, Gooley TA, Deeg HJ, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23:3439–3446. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 27.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 28.Cahn JY, Labopin M, Schattenberg A, et al. Allogeneic bone marrow transplantation for acute leukemia in patients over the age of 40 years. Acute Leukemia Working Party of the European Group for Bone Marrow Transplantation (EBMT) Leukemia. 1997;11:416–419. doi: 10.1038/sj.leu.2400573. [DOI] [PubMed] [Google Scholar]
- 29.Sutton L, Chastang C, Ribaud P, et al. Factors influencing outcome in de novo myelodysplastic syndromes treated by allogeneic bone marrow transplantation: a long-term study of 71 patients Societe Francaise de Greffe de Moelle. Blood. 1996;88:358–365. [PubMed] [Google Scholar]
- 30.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24:1050–1052. doi: 10.1038/leu.2010.12. [DOI] [PubMed] [Google Scholar]
- 31.Andersson BS, Valdez BC, de Lima M, et al. Clofarabine+/-Fludarabine with Once Daily IV Busulfan as Pretransplant Conditioning Therapy for Advanced Myeloid Leukemia and MDS. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.09.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell JA, Irish W, Balogh A, et al. The addition of 400 cGY total body irradiation to a regimen incorporating once-daily intravenous busulfan, fludarabine, and antithymocyte globulin reduces relapse without affecting nonrelapse mortality in acute myelogenous leukemia. Biol Blood Marrow Transplant. 2010;16:509–514. doi: 10.1016/j.bbmt.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]


