Abstract
In animal models, hypoxic pre-conditioning confers protection against subsequent neurological insults, mediated in part through an extensive vascular remodeling response. In light of the therapeutic potential of this effect, the goal of this study was to establish the dose-response relationship between level of hypoxia and the extent of cerebrovascular modeling, and to define the mildest level of hypoxia that promotes remodeling. Mice were exposed to different levels of continuous hypoxia (8-21% O2) for seven days before several aspects of vascular remodeling were evaluated, including endothelial proliferation, total vascular area, arteriogenesis, and fibronectin/α5β1 integrin expression. For most events, the threshold level of hypoxia that stimulated remodeling was 12-13% O2. Interestingly, many parameters displayed a biphasic dose-response curve, with peak levels attained at 10% O2, but declined thereafter. Further analysis in the 12-13% O2 range revealed that vascular remodeling occurs by two separate mechanisms: (i) endothelial hyperplasia, triggered by a hypoxic threshold of 13% O2, which leads to increased capillary growth, and (ii) endothelial hypertrophy, triggered by a more severe hypoxic threshold of 12% O2, which leads to expansion of large vessels and arteriogenesis. Taken together, these results define the hypoxic thresholds for vascular remodeling in the brain, and point to two separate mechanisms mediating this process.
Keywords: angiogenesis, arteriogenesis, endothelial cells, dose-response, hypoxic threshold, vascular remodeling, blood-brain barrier (BBB), α5 integrin, fibronectin
INTRODUCTION
Many neurological diseases have vascular dysfunction either as the root or as the central part of the pathogenic process. These include ischemic stroke (del Zoppo and Hallenbeck 2000; Dirnagl et al. 1999), multiple sclerosis (MS) (Gay and Esiri 1991; Kirk et al. 2003) and vascular dementia (Brown et al. 2009; Zlokovic 2011). Despite all the intensive research efforts in these different conditions, no current drugs or therapies have been identified that target the vascular origin.
Interestingly, over the last decade, a number of studies have demonstrated the protective potential of hypoxic pre-conditioning, in which a period of training at a sub-clinical hypoxic level protects against subsequent neurological sequelae (Dirnagl et al. 2003). This has been most effectively shown in ischemic stroke in which a short (2-4 hour) exposure to 8-10% hypoxia reduces the size of ischemic lesion and subsequent inflammation if ischemia occurs within 2-3 days of pre-conditioning (Dunn et al. 2012; Miller et al. 2001). In addition, a recent study suggests that more long-term hypoxic preconditioning might also protect against inflammatory demyelinating disease in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE), in part by limiting leukocyte infiltration (Dore-Duffy et al. 2011).
Interestingly, chronic mild hypoxia (CMH) induces beneficial physiological adaptations in cerebral vessels that work in the opposite direction to age-related deterioration, by promoting angiogenic and arteriogenic remodeling, thus increasing vessel density, blood-brain barrier (BBB) integrity and cerebral blood flow (LaManna et al. 2004; LaManna et al. 1992; Li et al. 2010). It is well established that mice exposed to CMH (8% O2 over a two week period), show greater than 50% increased vascular density in all areas of the brain. We have shown that this response involves active angiogenic remodeling underscored by endothelial cell proliferation driven by upregulation of the fibronectin-α5β1 integrin axis (Li et al. 2012; Milner et al. 2008). Significantly, CMH-induced vascular remodeling is not just limited to capillaries; it also involves robust generation of new arterioles (arteriogenesis), consistent with the idea that mild hypoxia stimulates remodeling at all stages of the vascular tree (Boroujerdi et al. 2012). Furthermore, cerebral blood vessels in hypoxic-exposed mice show strong upregulation of tight junction proteins, including claudin-5 and zonula occludens-1 (ZO-1) (Li et al. 2010), suggesting that CMH also promotes integrity of the BBB, the cellular barrier that protects the sensitive neuropil from the potentially hazardous components of blood (Ballabh et al. 2004; Pardridge 2003).
Our studies over the last five years have defined the time-course of cerebrovascular remodeling events in response to a standard hypoxic regimen of 8% O2 (Boroujerdi et al. 2012; Li et al. 2010). To our knowledge, no studies have examined the dose-response relationship between level of hypoxia and extent of vascular remodeling within the brain. More importantly, the threshold level of hypoxia that induces these changes has not yet been defined. This is an important issue because most animal studies to date have employed fairly severe levels of hypoxia, e.g.: 8% O2, which is equivalent to an altitude of 23,500 feet, conditions that are not practical or tolerable for most humans (Kupper et al. 2011). So before considering the clinical potential of this approach in human patients, it is important to define the mildest level of hypoxia that promotes beneficial remodeling. With this in mind, the goal of this study was to define the dose-response relationship between level of hypoxia and vascular remodeling in the mouse brain, and specifically to identify the hypoxic threshold at which vascular remodeling changes occur. As vascular remodeling encompasses a large number of distinct events, we focused specifically on: (i) endothelial cell proliferation, (ii) increase in total vascular area, (iii) vessel size distribution, (iv) arteriogenesis, (v) upregulated expression of the remodeling proteins fibronectin and its receptor α5β1 integrin, and (vi) upregulated expression of the tight junction protein claudin-5.
MATERIALS AND METHODS
Animals
The studies described have been reviewed and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. Wild-type C57Bl/6 mice were maintained under pathogen-free conditions in the closed breeding colony of The Scripps Research Institute (TSRI).
Chronic Hypoxia Model
Wild-type C57Bl/6 littermate mice, 8-10 weeks of age, were housed 4 to a cage, and placed into a hypoxic chamber (Biospherix, Redfield, NY) for 7 days maintained at different oxygen levels of 8, 10, 12, 14, and 16% O2. Control mice were kept in the same room under similar conditions except that they were kept at ambient sea-level oxygen levels (normoxia, approximately 21% O2 at sea-level) for the duration of the experiment. In subsequent experiments, mice were maintained at 8% and 13% O2 for periods of 7 and 14 days. Every few days, the chamber was briefly opened for cage cleaning and food and water replacement as needed.
Immunohistochemistry and antibodies
Immunohistochemistry was performed as described previously (Milner and Campbell 2002) on 10 μm frozen sections of cold phosphate buffer saline (PBS) perfused brains taken from mice subject to either normoxia (control) or hypoxic conditions. The following monoclonal antibodies were obtained from BD Pharmingen (La Jolla, CA): rat monoclonal antibodies reactive for: CD31 (clone MEC13.3), CD 105 (clone MJ7/18), and the integrin subunit α (clone 5H10-27 (MFR5)). Other antibodies used included: rabbit anti-fibronectin (Sigma, St. Louis, MO), mouse anti-α-SMA-Cy3 conjugate (Sigma, clone 1A4), rabbit anti-claudin-5 (Invitrogen, Carlsbad, CA), and rabbit anti-Ki67 (Vector laboratories, Burlingame, CA). Secondary antibodies used included goat anti-rabbit Cy3 (Jackson Immunoresearch, Baltimore, PA) and anti-rat Alexa Fluor 488 (Invitrogen).
Image analysis
Images were taken using a 20X objective on a Zeiss Imager M1.m. All analysis was performed in the frontal lobe region of the brain. For each antigen, three images were taken per region at 10X or 20X magnification and the mean calculated for each subject over three different sections. All data analysis was performed using the Perkin Elmer Volocity software (Waltham, MA). This includes quantification of total vessel (CD31-positive) area, numbers of CD31/Ki67 dual-positive cells, α-SMA-positive vessels, α5 integrin-positive vessels, low intensity CD105 vessels and the size distribution of vessels. To quantify the mean expression levels of vascular fibronectin, α5 integrin, CD105, and claudin-5, Volocity software was used to measure the fluorescent intensity of each vessel, and thereby calculate the mean vessel intensity per field of view for each hypoxic condition. Each experiment was performed with four different animals per condition, and the results expressed as the mean ± SEM. Statistical significance was assessed by using the Student's t test, in which p < 0.05 was defined as statistically significant.
RESULTS
The threshold level of hypoxia that stimulates vascular remodeling in the brain lies between 12-14% O2
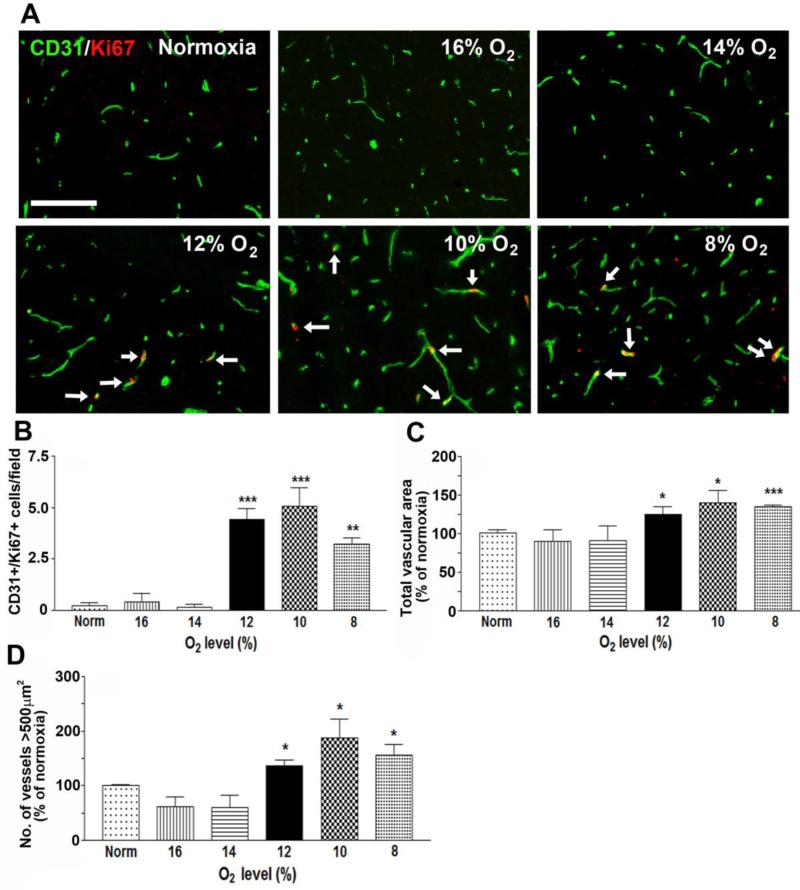
To define the dose-response relationship between hypoxia and cerebrovascular remodeling, mice were incubated for seven days either under control (normoxic) conditions or at different levels of hypoxia including 16, 14, 12, 10 and 8% O2. In previous studies we have shown that hypoxic-induced vascular remodeling employing a hypoxic level of 8% O2 occurs across all areas of the brain (Milner et al. 2008). In the current study we focused our attention specifically on the frontal lobe as this is a large structure with well-defined vascular architecture. We chose the seven day period for two reasons. First, this is the time-point at which many of the remodeling parameters show peak levels, including fibronectin and α5β1 integrin expression, endothelial proliferation, and the number of low-CD105 vessels as a marker of arteriogenesis (Boroujerdi et al. 2012; Li et al. 2010). Second, seven days is sufficient time to detect significant differences in total vascular area. All changes in vascular remodeling were evaluated by immunofluorescence (IF) and digital software analysis using several well-defined measures that are altered during hypoxia-induced cerebrovascular remodeling. To start with, we examined three parameters of vascular remodeling that show clear changes in response to hypoxia: endothelial cell proliferation (CD31/Ki67), total vessel area (CD31), and the number of large area vessels (>500μm2).
As shown in Figure 1, significant increases in endothelial cell proliferation (identified by CD31/Ki67 dual-positive cells) were not observed until ambient O2 levels reached 12% or lower. Compared with normoxic (control) conditions (0.15 ± 0.1 proliferating cells per field of view), endothelial cell proliferation was significantly increased at O2 levels of 12% (4.4 ± 0.5 proliferating cells per field of view, p < 0.005), 10% (5.1 ± 0.9 proliferating cells per field of view, p < 0.005), and 8% (3.2 ± 0.3 proliferating cells per field of view, p < 0.01) (Figure 1B). In a similar manner, alterations in total vascular area were not observed until ambient O2 levels were 12% or lower (Figure 1C). Compared with normoxic (control) conditions, total vascular area was significantly higher at O2 levels of 12% (125 ± 9.5%, p < 0.05), 10% (141.3 ± 15.1%, p < 0.05), and 8% (134.5 ± 2.3%, p < 0.005).
Figure 1.
The influence of hypoxic dose on vascular remodeling in the brain. A. Dual-IF was performed on frozen sections of the frontal lobe from mice exposed to different hypoxic levels (8-16% O2) or normoxia for 7 days using antibodies specific for CD31 (AlexaFluor-488, green) or Ki67 (Cy3, red). Scale bar = 100μm. Arrows denote the Ki67+ endothelial cells. B-D. Quantification of the number of CD31/Ki67dual-positive cells (B), total vessel area (C), or the number of vessels with area >500μm2 (D). All experiments were performed with four different animals per condition, and the results expressed either as the mean ± SEM of dual-positive cells (B) or as the mean ± SEM of the % change compared to normoxic conditions (C-D). Note that increases in all three parameters (endothelial cell proliferation, total vascular area and number of large area vessels) were not observed until hypoxic levels reached 12% O2 or lower. * P < 0.05, ** P < 0.01, ***P < 0.005.
In previous studies our lab and the Moreno lab independently demonstrated that one of the most striking effects of CMH is an increase in the number of large area vessels (>500μm2) (Boroujerdi et al. 2012; Freitas-Andrade et al. 2011). When we examined the dose-response of this effect, we found that hypoxic-induced increases in the number of large area vessels were not observed until ambient O2 levels were 12% or lower (Figure 1D). Compared with normoxic (control) conditions, the number of large area vessels was significantly higher at O2 levels of 12% (135.8 ± 10.9%, p < 0.05), 10% (187 ± 35.5%, p < 0.05), and 8% (156.5 ± 19.2%, p < 0.05). Thus, for all three parameters examined, the threshold hypoxic level that stimulated vascular remodeling appeared to lie somewhere in the range 12-14% O2. Surprisingly, in all of the parameters examined, the extent of remodeling at 8% O2 appeared to be less than that at 10% O2, suggesting the presence of a biphasic relationship between hypoxia and vascular remodeling. This implies that 10% O2 promotes the maximal level of vascular remodeling, but at more hypoxic levels, vascular remodeling actually declines.
In a recent study, we showed that CMH promotes an active arteriogenic response in the brain (Boroujerdi et al. 2012). We also made the novel observation that actively remodeling arterioles can be identified by their reduced endothelial expression of CD105, an auxiliary receptor for transforming growth factor (TGF)-β. Therefore, to examine the relationship between hypoxic dose and arteriogenic response, we quantified the number of α-SMA-positive-positive vessels. This revealed that the number of α-SMA-positive vessels only started to increase at ambient O2 levels of 12% or lower (Figure 2B). Compared with normoxic (control) conditions, the number of α-SMA-positive vessels was significantly higher at O2 levels of 12% (173 ± 22.3%, p < 0.05), 10% (166 ± 22.8%, p < 0.05), and 8% (187 ± 25%, p < 0.05). In addition, as CD105 expression is markedly reduced on actively remodeling arterioles (Boroujerdi et al. 2012), we used CD105 expression as a marker to define the hypoxic level that triggers arteriogenic remodeling. In this approach we quantified mean CD105 expression levels on all arterioles (α-SMA-positive vessels) and also non-arterioles (α-SMA-negative vessels = all other vessels) at the different hypoxic levels. As shown in Figure 2A, this confirmed that under normoxic conditions, CD105 expression on α-SMA-positive arterioles (arrow) was higher than smaller vessels. By contrast, under hypoxic conditions of 8% O2, CD105 expression on arterioles was markedly reduced (arrows). Arterial CD105 levels were also reduced at 12% O2, though to a lesser extent (arrows). Quantification of this switch (Figure 2C) demonstrated that at increasing levels of hypoxia, CD105 expression on arterioles (α-SMA-positive vessels, black bars) declined, while expression on all other vessels (α-SMA-negative vessels, white bars) increased. Of note, these changes only showed statistical significance at ambient O2 levels of 12% or lower. This shows that the hypoxic threshold for arteriogenic remodeling also lies between 12-14% O2.
Figure 2.
The influence of hypoxic dose on arterial remodeling in the brain. A. Dual-IF was performed on frozen sections of the frontal lobe from mice exposed to different hypoxic levels (8-16% O2) or normoxia for 7 days using antibodies specific for CD105 (AlexaFluor-488, green) or α-SMA (Cy3, red). Scale bar = 100μm. B. Quantification of the number of α-SMA-positive vessels at different levels of hypoxia. C. Quantification of CD105 expression levels on α-SMA-positive vessels (arterioles) or α-SMA-negative vessels (all other vessels) at different levels of hypoxia. All experiments were performed with four different animals per condition, and the results expressed as the mean α SEM of the % change compared to normoxic conditions. Note that increases in arterial density were not observed until hypoxic levels reached 12% O2 or lower (panel B). Also note that under normoxic conditions, CD105 levels were higher on α-SMA-positive vessels than on other vessels (arrow in panel A), but at levels of hypoxia 12% O2 or lower, this pattern reversed, with CD105 expression levels increased on α-SMA-negative vessels but reduced on α-SMA-positive vessels (arrows in panel A, and quantified in panel C). * P < 0.05, ** P < 0.01.
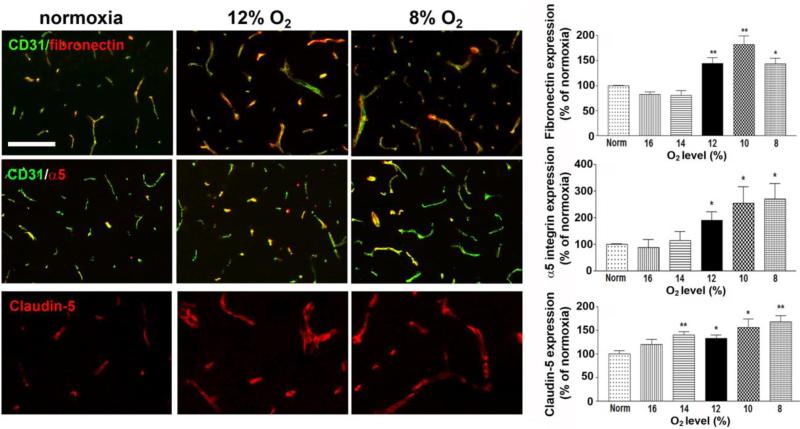
The threshold level of hypoxia that stimulates upregulation of the angiogenic proteins fibronectin and α5 integrin expression also lies between 12-14% O2
In previous work, we demonstrated an important role for the fibronectin-α5β1 integrin axis in driving endothelial cell proliferation and angiogenic remodeling in the hypoxic brain (Li et al. 2012), so next we examined how expression of this important angiogenic axis is regulated by hypoxic dose. Analysis of the dose-response relationship between hypoxia and vascular expression of fibronectin and α5 integrin revealed that both proteins begin to get significantly upregulated at O2 levels of 12% or lower (Figure 3). Vascular fibronectin levels at 12% O2 (144 ± 11.4%, p < 0.01), 10% O2 (181.5 ± 17.9%, p < 0.01), and 8% O2 (148.5 ± 13.7%, p < 0.05) were all significantly increased over the control (normoxic) value. In a similar way, endothelial α5 integrin levels at 12% O2 (190.3 ± 32.1%, p < 0.05), 10% O2 (254 ± 62.6%, p < 0.05), and 8% O2 (269.5 ± 58.2%, p < 0.05) were all significantly increased over the control (normoxic) value. Interestingly, in a similar manner to other vascular remodeling parameters, fibronectin expression also showed a biphasic pattern, with the highest level of fibronectin expression recorded at 10% O2, and lower levels observed at 8% O2.
Figure 3.
The influence of hypoxic dose on the expression of angiogenic and tight junction proteins on cerebral vessels. Dual-IF was performed on frozen sections of the frontal lobe from mice exposed to different hypoxic levels (8-16% O2) or normoxia for 7 days using antibodies specific for CD31 or fibronectin, CD31 or α5 integrin, or claudin-5. Scale bar = 100μm. Quantification of the changes in protein expression level are displayed in graphs. All experiments were performed with four different animals per condition, and the results expressed as the mean ± SEM of the % change compared to normoxic conditions. Note that increases in vascular fibronectin and α5 integrin expression were not observed until hypoxic levels reached 12% O2 or lower. In contrast, claudin-5 expression levels were significantly increased at the milder hypoxic level of 14% O2. * P < 0.05, ** P < 0.01.
The threshold level of hypoxia that stimulates upregulation of claudin-5 expression at the BBB occurs at a milder hypoxic level than other parameters, between 14-16% O2
Previously we demonstrated that CMH promotes strong upregulation of two tight junction proteins on cerebral blood vessels, claudin-5 and ZO-1, suggesting that CMH may promote BBB integrity (Li et al. 2010). Up until this point, all remodeling parameters we examined showed a hypoxic threshold between 12-14% O2. Interestingly, when we examined claudin-5 expression, we noticed that the hypoxic threshold required to trigger significant increases in claudin-5 expression occurred at a milder dose of hypoxia than for other markers. In the case of claudin-5, expression levels were significantly increased at O2 levels of 14% or less (Figure 3). Compared with normoxic (control) conditions, claudin-5 expression was significantly higher at O2 levels of 14% (140.3 ± 6.6%, p < 0.01), 12% (132.5 ± 6.8%, p < 0.05), 10% (155.5 ± 18.8%, p < 0.05), and 8% (168 ± 12.8%, p < 0.01). This demonstrates that endothelial claudin-5 expression appears to be more sensitive to the influence of hypoxia than other vascular parameters.
Fine-tuning experiments suggest that vascular remodeling occurs through two separate mechanisms
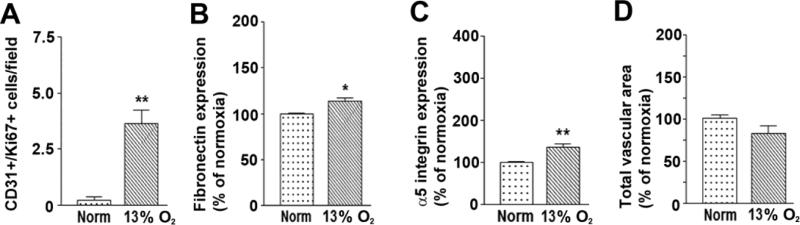
In our first series of experiments we employed different levels of hypoxia, separated by 2% increments. Having determined that the critical hypoxic threshold that stimulates vascular remodeling lies somewhere in the range 12-14% O2, we next wanted to more precisely define the hypoxic threshold level, to determine whether the hypoxic threshold was actually 12% O2, or perhaps closer to 13% O2. To examine this, we performed a similar analysis using a new hypoxic level of 13% O2. As shown in Figure 4, this revealed that a hypoxic level of 13% O2 was also highly effective at promoting endothelial cell proliferation (3.6 ± 0.6 proliferating cells per field of view vs. 0.15 ± 0.1 cells under normoxic conditions, p < 0.01, Figure 4A), upregulating vascular fibronectin expression (113.3 ± 3.9% of the control value, p < 0.05, Figure 4B) and upregulating endothelial α5 integrin expression (135.7 ± 6.8% of the control value, p < 0.01, Figure 4C). Thus all these vascular remodeling parameters were triggered by a hypoxic threshold of 13% O2. However unexpectedly, a hypoxic level of 13% O2 did not increase the total vascular area (Figure 4D). So while a hypoxic level of 13% O2 increased expression of the fibronectin-α5β1 integrin axis, thus promoting endothelial proliferation, this level of hypoxia did not result in increased total vascular area. This came as a big surprise to us as up to this point, we had worked on the assumption that endothelial proliferation was a major contributor to overall vascular expansion and increased vascular area (Li et al. 2012; Li et al. 2010). However, this new data suggests that endothelial cell proliferation and increased total vascular area may not always be directly linked.
Figure 4.
Defining the precise hypoxic threshold of vascular remodeling in the brain. Quantification of the number of CD31/Ki67 dual-positive cells (A), total vessel area (B), mean expression level of vascular fibronectin (C), or mean expression level of vascular α5 integrin (D). All experiments were performed with four different animals per condition, and the results expressed either as the mean ± SEM of dual-positive cells (A) or as the mean ± SEM of the % change compared to normoxic conditions (B-D). Note that increases in all vascular remodeling parameters were not observed until hypoxic levels reached 13% O2 or lower, though in the case of total vascular area, significance was not reached until hypoxia was 12% O2 or lower. * P < 0.05, ** P < 0.01, ***P < 0.005.
At this point, we started to look more closely at the location of the proliferating endothelial cells within the vascular tree. What we observed is that most proliferating endothelial cells were located within small diameter capillaries (Figure 5A). They were almost never found within larger diameter vessels and they never co-localized with α-SMA-positive arterioles (Figure 5B). Based on this information, we hypothesized that new capillary formation and expansion of larger diameter arterial vessels might be two separate events. According to this hypothesis, the milder hypoxic stimulus of 13% O2 would promote endothelial proliferation and increase the number of small area vessels (capillaries), but would not result in increased numbers of larger area vessels, and thus have minimal impact on the total vascular area.
Figure 5.
Endothelial proliferation is not directly linked to an increased number of large area vessels. A-B. Hypoxic-induced endothelial proliferation is restricted to capillaries. Dual-IF was performed on frozen sections of the frontal lobe from mice exposed to 8% O2 hypoxia for 7 days using antibodies specific for CD31 or Ki67 (A) or α-SMA or Ki67 (B). Scale bar = 50μm. Note that proliferating endothelial cells were located within small diameter capillaries, but they were never found within larger diameter vessels (A) or α-SMA-positive arterioles (B). C. The influence of different levels of hypoxia on vessel size distribution following vascular remodeling. Frozen sections of frontal lobe taken from mice exposed to different hypoxic levels (8-16% O2) or normoxia for 7 days were immunostained for CD31, photographed and vessel size distribution analysis performed using Volocity software. All points represent the mean ± SEM of four subjects. Note that 13% O2 marginally increased the number of small area vessels, but did not increase the number of large area (> 500μm2) vessels. In contrast, the slightly more hypoxic level of 12% O2 significantly increased the number of large area (> 500μm2) vessels. * P < 0.05, ** P < 0.01.
To test this idea, we examined the vessel size distribution at different levels of hypoxia. Our previous studies have shown that chronic hypoxia at a level of 8% O2 promotes a right-shift in the vessel size distribution, so that the number of smallest area vessels is reduced while the number of larger area vessels is increased (Boroujerdi et al. 2012). If our hypothesis is correct, it would predict that at 13% O2, the number of small area vessels would increase, but there would be little or no change in the large area vessels. In fact when we examined the size distribution data, this is exactly what we saw. As shown in Figure 5C, 13% O2 resulted in a trend towards increased numbers of small area vessels (0-100μm2), consistent with increased endothelial proliferation, but this milder level of hypoxia did not increase the number of large area vessels (>500μm2). As larger area vessels make the greatest contribution to total vascular area, this fits with our observation of a lack of 13% O2 increasing total vascular area. In contrast, as shown in Figures 1B-D, the slightly more hypoxic level of 12% O2, as well as promoting endothelial proliferation, also resulted in increased numbers of large area vessels and increased total vascular area. Taken together, these findings support the idea that vascular remodeling in the hypoxic brain occurs through two distinct processes that have different hypoxic thresholds: (i) an endothelial hyperplasia response, in which endothelial proliferation (triggered by a hypoxic threshold of 13% O2) leads to increased capillary growth, and (ii) an endothelial hypertrophic response, in which vascular enlargement and arteriogenesis (triggered by a more severe hypoxic threshold of 12% O2) occurs independently of endothelial proliferation.
The time-course of the remodeling response is independent of the strength of the hypoxic stimulus
In previous studies we have shown that in response to a chronic mild hypoxic (CMH) stimulus of 8% O2, the peak level of endothelial proliferation and fibronectin expression occurs between 4-7 days (Li et al. 2010; Milner et al. 2008). One thing that is important to examine is whether the time-course of the vascular remodeling response is altered by the strength of the hypoxic stimulus. Specifically, we wondered if a milder hypoxic stimulus might result in a delayed response with an extended time-course. To address this question, we compared hypoxic levels of 8% O2 and 13% O2 and then examined endothelial cell proliferation and fibronectin expression levels after 7 and 14 days of hypoxia. As shown in Figure 6, at both levels of hypoxia, endothelial cell proliferation and vascular fibronectin expression levels both peaked at the 7 day time-point, and both had returned to baseline levels after 14 days. This suggests that hypoxic-induced vascular remodeling follows a similar time-course regardless of the dose of hypoxic stimulus.
Figure 6.
The time-course of the vascular remodeling response is independent of hypoxic dose. Mice were exposed to normoxia or hypoxic levels of 8% or 13% O2 for 7 days or 14 days and then analyzed by CD31/Ki67 dual-IF to define the number of proliferating endothelial cells (A) or by CD31/fibronectin IF to determine changes in vascular fibronectin expression (B). All experiments were performed with four different animals per condition, and the results expressed either as the mean ± SEM of dual-positive cells (A) or as the mean ± SEM of the % change compared to normoxic conditions (B). Note that at both levels of hypoxia, endothelial cell proliferation and level of fibronectin expression both peaked at the 7 day time-point, but had returned to baseline levels after 14 days. * P < 0.05, ** P < 0.01.
DISCUSSION
Hypoxic pre-conditioning confers strong protection against subsequent neurological insults (Dore-Duffy et al. 2011; Dunn et al. 2012; Miller et al. 2001). As mild hypoxia also stimulates an extensive vascular remodeling response that increases brain vascularity (LaManna et al. 1992), promotes tight junction protein expression at the BBB (Li et al. 2010), and reduces inflammatory leukocyte adhesion and extravasation across the BBB (Dore-Duffy et al. 2011; Stowe et al. 2011), it seems likely that at least some of the protection conferred by hypoxic pre-conditioning is a result of altered vascular structure and function. Therefore, before considering the therapeutic potential of hypoxic preconditioning in human patients, an important first step is to establish the dose-response relationship between hypoxia and vascular modeling, so as to define the mildest level of hypoxia that promotes beneficial remodeling. To achieve this goal, here we examined vascular remodeling in the brains of mice exposed to different levels of hypoxia, and defined the hypoxic threshold at which vascular remodeling occurs. There were four main conclusions from this study. First, the threshold level of hypoxia that stimulated vascular remodeling events occurred in the range 12-13% O2. Second, for many parameters examined, including endothelial proliferation and fibronectin expression, the dose-response curve was biphasic, with peak levels attained at the 10% O2 level, but declined by 8% O2. Third, careful analysis in the hypoxic threshold range (12-13% O2) revealed that vascular remodeling in the brain occurs by two separate processes: (i) an endothelial hyperplasia response which leads to increased capillary growth, and (ii) an endothelial hypertrophic response which leads to dilatation of large vessels and arteriogenesis. Fourth, the time-course of vascular remodeling was found to be independent of the strength of the hypoxic stimulus.
The significance of defining hypoxic thresholds and hypoxic-remodeling dose-response curves
While it has been known for more than twenty years that mild hypoxia at the level of 8-10% O2 triggers a strong vascular remodeling response in the rodent CNS (LaManna et al. 1992), to our knowledge, no studies have ever defined the dose-response or identified the threshold level of hypoxia that induce these changes. Our studies were motivated by the idea that hypoxic preconditioning may provide a useful therapeutic tool for patients suffering from, or at risk of, serious neurological disease. Before even considering this approach for clinical studies, it is an important first step to define the hypoxic thresholds at which these events occur in rodents. Interestingly, our results demonstrate that significant vascular remodeling occurs in the brain of mice at much milder hypoxic levels (12-13% O2 equivalent to 10,340-12,370 feet altitude) than those typically used in standard mouse models of CMH (8% O2 equivalent to 23,500 feet altitude). This has important implications when considering the future clinical potential of RIMH, because it means that in practice, patients would not need to be exposed to such challenging hypoxic conditions. Instead, milder hypoxic levels (12-13% O2) would be sufficient to promote therapeutically beneficial adaptive responses. Unexpectedly, our findings also point to a biphasic relationship between hypoxic dose and strength of remodeling response. Maximal levels of many remodeling parameters were reached at a hypoxic level of 10% O2, but then declined at 8% O2. This suggests that the molecular mechanisms responsible for hypoxic-induced physiological adaptation begin to fail at hypoxic levels more severe than 10% O2. The most likely reason for this is that inadequate oxygen levels trigger pathological mechanisms that over-ride the physiological adaptive mechanisms, leading to an overall deleterious outcome. These might include excess production of oxygen free radicals or induction of pro-inflammatory cytokines such as interferon-gamma, which has well-described anti-angiogenic effects (Sidky and Borden 1987).
Animals exposed to chronic hypoxia undergo a wide range of physiological adaptations, which include hyperventilation, increased cerebral blood flow and elevated hematocrit. In the short term, environmental hypoxia leads to reduced arterial partial pressure of O2 (PaO2) as well as reduced PaCO2 (Ainslie and Ogoh 2009; LaManna et al. 2004). With this in mind, it is important to consider that the vascular remodeling observed under hypoxic conditions may be a direct result of hypoxia or it could be induced by one of the physiological compensatory mechanisms, or a combination of both. In these studies we made the interesting observation that hypoxia had opposite effects on CD105 expression on different types of vessel; decreasing CD105 expression on arterioles but increasing expression on capillaries. While these results are consistent with previous studies (Boroujerdi et al. 2012; Minhajat et al. 2006; Yao et al. 2005), the mechanisms that regulate this expression have yet to be defined.
Differential sensitivity of endothelial tight junction protein expression
One clear message to emerge from these studies is that while 12-13% O2 appears to be the critical hypoxic threshold at which most vascular remodeling events are triggered, upregulation of the endothelial tight junction protein claudin-5 occurred at even milder levels of hypoxia (between 14-16% O2). This suggests that the homeostatic mechanism responsible for maintaining BBB integrity is acutely sensitive to the levels of hypoxia. Considering that BBB breakdown is an early event in several neurological conditions, including MS and ischemic stroke (del Zoppo et al. 1986; del Zoppo and Hallenbeck 2000; Gay and Esiri 1991), this finding has strong clinical implications because it offers the possibility of using relatively mild levels of hypoxia as a means of selectively increasing tight junction expression and thus BBB integrity, without triggering widespread vascular remodeling changes. Interestingly, a number of studies using pathological levels of hypoxia (6% O2) have shown that under some circumstances, increased expression of tight junction proteins may actually reflect barrier dysfunction (McCaffrey et al. 2009; Willis et al. 2010). Thus in future studies, it will be important to evaluate the precise relationship between endothelial tight junction protein expression and BBB integrity, over a range of hypoxic levels using a number of tracer molecules of different molecular weight.
Two distinct mechanisms driving vascular remodeling in the hypoxic brain
In the current study we were surprised to find that endothelial proliferation wasn't directly linked to increased total vascular area. This first hint of this was suggested by our observation that a mild hypoxic stimulus of 13% O2 promoted endothelial proliferation, leading to an increased number of small area vessels, but did not increase the number of large area vessels or total vascular area. By contrast, the slightly stronger hypoxic dose of 12% O2 promoted endothelial proliferation, and also resulted in an increased number of large area vessels, resulting in increased total vascular area. To follow up this apparent un-coupling between endothelial proliferation and increased total vascular area, we started to look more closely at the location of proliferating endothelial cells within the vascular tree. This revealed that most proliferating endothelial cells were located within small diameter capillaries, but significantly they were never found within larger diameter vessels and they never co-localized with α-SMA-positive arterioles. Together, these findings support the idea that vascular remodeling in the hypoxic brain occurs through two distinct processes that have different hypoxic thresholds: (i) an endothelial hyperplasia response in which endothelial proliferation (triggered by a hypoxic threshold of 13% O2) leads to increased capillary growth, and (ii) an endothelial hypertrophic response in which vascular dilatation and arteriogenesis, (triggered by a more severe hypoxic threshold of 12% O2) occurs independently of endothelial proliferation. The separate processes of angiogenesis and arteriogenesis are known to be regulated by different factors. Angiogenesis is triggered by hypoxia inducible factor-1-alpha (HIF-1α) and a cascade of downstream pathways that include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and angiopoietin-2 (Ang-2) (Chavez et al. 2000; Folkman 1995; Kuo et al. 1999; Pichiule et al. 1999; Pichiule and LaManna 2002). In contrast, arteriogenesis is triggered directly by hemodynamic factors such as stretch or flow shear stress (Persson and Buschmann 2011), and also by inflammatory mediators such as granulocyte monocyte-colony stimulating factor (GM-CSF) (Buschmann et al. 2003; Todo et al. 2008). Now that we have defined different hypoxic thresholds for the separate processes of angiogenesis (13% O2) and arteriogenesis (12% O2), it should be feasible to determine which of the two are most important for the vascular pre-conditioning response. In the planned experiment, mice will be exposed to hypoxic levels no less than 13% O2. Based on our current data, we predict that this level of hypoxia will confer benefit by increasing capillary density and tight junction protein expression. It is somewhat harder to predict the importance of arteriogenesis in the pre-conditioning response, but these experiments will shed important new light on this question.
Clinical implications of these findings
While the protective effect of hypoxic pre-conditioning on ischemic stroke has been established for some time, this was based on studies using single brief hypoxic exposures which conferred protection from ischemic events for several days (Dunn et al. 2012; Miller et al. 2001). More recently, a key study demonstrated that the period of protection from ischemia could be extended from days to months by repeated bouts of intermittent hypoxia (Stowe et al. 2011). This important finding opens up the idea that repeated exposure to mild hypoxia might provide one way of developing long-term protection from ischemic events. Extending this idea, the goal of the current study was to define hypoxia-vascular remodeling dose-response relationships and more importantly, identify the hypoxic threshold levels that might be useful in such a strategy. Here we have demonstrated that the hypoxic threshold (or dose) for inducing vascular remodeling in the brain is 12-13% O2, and for increasing endothelial tight junction protein expression is 14-16% O2. When considering any treatment protocol, three parameters of the treatment need to be considered: dose, duration and frequency. Having identified the threshold level (or dose) of continuous hypoxia that promotes vascular remodeling, in the next set of studies, we aim to define the minimal daily duration of hypoxia required to stimulate vascular remodeling. With this information in hand, the stage will be set to start to perform pre-clinical experiments, to examine the role of regular intermittent mild hypoxia (RIMH) in the prevention and/or treatment of a range of neurological disorders including ischemic stroke and MS. Based on recent encouraging data showing that intermittent mild hypoxia (1-2 hours/day) promoted several aspects of brain function in mice, including hippocampal neurogenesis, anti-depressant activity and protection from mitochondrial stress due to ethanol withdrawl (Ju et al. 2012; Stowe et al. 2011; Zhu et al. 2010) this promises to be a fruitful avenue of research.
Highlights.
Examined the dose-response between hypoxia and vascular remodeling in mouse brain
The hypoxic threshold that stimulated vascular remodeling occurred at 12-13% O2
The curve was biphasic, with peak levels attained at 10% O2, but declined by 8% O2
Remodeling occurs by 2 separate processes endothelial hyperplasia and hypertrophy
The time-course of remodeling was independent of the strength of hypoxic stimulus
ACKNOWLEDGMENTS
This work was supported by a Postdoctoral Fellowship Award from the American Heart Association (AB) and by the NIH RO1 grant NS060770 (RM). This is manuscript number 27076 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Experimental Physiology. 2009;95:251–262. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview. Structure, regulation and clinical implications. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Boroujerdi A, Welser-Alves J, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in β1 integrins. J Cereb Blood Flow Metab. 2012;32:1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white matter in dementia. J Neurol Sci. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann IR, Busch H-J, Mies G, Hossmann K-A. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxic inducible factor 1α in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis Research. 2000;98:V73–V81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathophysiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Wencel M, Katyshev V, Cleary K. Chronic mild hypoxia ameliorates chronic inflammatory activity in myelin oligodendrocyte glycoprotein (MOG) peptide induced experimental autoimmune encephalomyelitis (EAE). Adv Exp Med Biol. 2011;701:165–173. doi: 10.1007/978-1-4419-7756-4_23. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Wu Y, Zhao Z, Srinivasan S, Natah SJ. Training the brain to survive stroke. PLoS One. 2012;7:e45108. doi: 10.1371/journal.pone.0045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Freitas-Andrade M, Carmeliet P, Charlebois C, Stanimirovic DB, Moreno MJ. PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge. J Cereb Blood Flow Metab. 2011;32:663–75. doi: 10.1038/jcbfm.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Esiri M. Blood-Brain Barrier Damage in Acute Multiple Sclerosis Plaques. Brain. 1991;114:557–572. doi: 10.1093/brain/114.1.557. [DOI] [PubMed] [Google Scholar]
- Ju X, Mallet RT, Metzger DB, Jung ME. Intermittent hypoxia conditioning protects mitochondrial cytochrome c oxidase od rat cerebellum from ethanol withdrawal stress. J Appl Physiol. 2012;112:1706–1714. doi: 10.1152/japplphysiol.01428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junction abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Kuo N-T, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- Kupper T, Milledge JS, Hillebrandt D, Kubalova J, Hefti U, Basnyat B, Giesler U, Pullan R, Schoffl V. Work in hypoxic conditions-consensus statement of the medical commission of the Union Internationale des Associations d'Alpinisme (UIAA MedCom). Ann Occup Hyg. 2011;55:369–386. doi: 10.1093/annhyg/meq102. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- Li L, Welser-Alves JV, van der Flier A, Boroujerdi A, Hynes RO, Milner R. An angiogenic role for the α5β1 integrin in promoting endothelial cell proliferation during cerebral hypoxia. Exp Neurol. 2012;237:46–54. doi: 10.1016/j.expneurol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Welser JV, Dore-Duffy P, Del Zoppo GJ, LaManna JC, Milner R. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia. 2010;58:1157–1167. doi: 10.1002/glia.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110:58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 2001;12:1663–9. doi: 10.1097/00001756-200106130-00030. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O. Organ-specific endoglin (CD105) expression in the angiogenesis of human cancers. Pathology International. 2006;56:717–723. doi: 10.1111/j.1440-1827.2006.02037.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier drug targetting: the future of brain drug development. Mol Med. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- Persson AB, Buschmann IR. Vascular growth in health and disease. Fronts Molec Neurosci. 2011;4:1–15. doi: 10.3389/fnmol.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, Chávez JC, Xu K, LaManna JC. Vascular endothelial growth factor upregulation in transient global ischemia induced by cardiac arrest and resuscitation in rat brain. Brain Res Mol Brain Res. 1999;74:83–90. doi: 10.1016/s0169-328x(99)00261-2. [DOI] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and de-adaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor-and lymphocyte-induced vascular responses. Cancer Res. 1987;47:5155–61. [PubMed] [Google Scholar]
- Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol. 2011;69:975–985. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, Yagita Y, Hori M. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–82. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–59. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201–206. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature Reviews Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]