Abstract
The NIH/NIAID initiated a countermeasure program to develop mitigators for radiation-induced injuries from a radiological attack or nuclear accident. We have previously characterized and demonstrated mitigation of single organ injuries, such as radiation pneumonitis, pulmonary fibrosis or nephropathy by angiotensin converting enzyme (ACE) inhibitors. Our current work extends this research to examine the potential for mitigating multiple organ dysfunctions occurring in the same irradiated rats. Using total body irradiation (TBI) followed by bone marrow transplant, we tested four doses of X radiation (11, 11.25, 11.5 and 12 Gy) to develop lethal late effects. We identified three of these doses (11, 11.25 and 11.5 Gy TBI) that were lethal to all irradiated rats by 160 days to test mitigation by ACE inhibitors of injury to the lungs and kidneys. In this study we tested three ACE inhibitors at doses: captopril (88 and 176 mg/m2/day), enalapril (18, 24 and 36 mg/m2/day) and fosinopril (60 mg/m2/day) for mitigation. Our primary end point was survival or criteria for euthanization of morbid animals. Secondary end points included breathing intervals, other assays for lung structure and function and blood urea nitrogen (BUN) to assess renal damage. We found that captopril at 176 mg/m2/day increased survival after 11 or 11.5 Gy TBI. Enalapril at 18–36 mg/m2/day improved survival at all three doses (TBI). Fosinopril at 60 mg/m2/day enhanced survival at a dose of 11 Gy, although no improvement was observed for pneumonitis. These results demonstrate the use of a single countermeasure to mitigate the lethal late effects in the same animal after TBI.
INTRODUCTION
Accidental exposure to high doses of ionizing radiation often results in death due to multiple organ dysfunction. In our efforts to develop countermeasures for mitigating life-threatening radiation-induced damage to multiple organs, we have focused on the lungs and the kidneys, which are injured after total body irradiation (TBI) (1–3) and are some of the most sensitive organs that exhibit delayed injuries in survivors of the acute radiation hematopoietic and gastrointestinal (GI) syndromes (4–7). We previously developed a rat model of total body irradiation followed by a bone marrow transplant (BMT) using a 11 Gy single dose of X ray that did not cause gastrointestinal (GI) toxicity (8). Rats were followed to 100 days and damage to the lung was assessed from 42–80 days by multiple end points including histopathology. Survival as well as lung damage was mitigated by short-term treatment with the superoxidedismutase catalase mimetic EUK 207 (8). Moulder et al. (9) used a similar model with 8.9–12.2 Gy to study radiation-induced damage to the kidney. In those studies elevated blood urea nitrogen (BUN) levels were correlated with morbidity and confirmed as a surrogate marker of radiation nephropathy based on multiple end points including proteinurea and renal histology. Renal dysfunction with proteinurea and BUN levels .20 mg/dl were observed at 30–35 days after 10 Gy TBI (10). Radiation nephropathy progressed until rats reached the IACUC criteria for euthanization (around 140 days). Rats were seldom euthanized before 100 days after doses ≥10 Gy TBI/BMT (9, 10), indicating that these doses did not induce lethality due to radiation pneumonitis, which occurred before 80 days (8).
We have previously mitigated2 localized thoracic radiation pneumonitis by ACE inhibitors captopril and enalapril but not fosinopril. In those studies lungs were injured by irradiation to the thorax only (10–13 Gy). While no rats were lost to pneumonitis after exposure to 10 Gy, between 70–90% of the animals met the IACUC criteria for euthanization after exposure to 13 Gy. Radiation nephropathy after a single dose of 10 Gy TBI followed by BMT was mitigated by captopril (9). Since ACE inhibitors mitigate radiation injuries to both the lungs and kidneys independently, we tested efficacy to mitigate lethal pneumonitis and/or nephropathy in the same rats after 11 Gy TBI/BMT and with higher doses of radiation (11.25 or 11.5 Gy TBI/BMT). In an effort to optimize the dose of ACE inhibitors we then tested the best mitigators from the 11 Gy TBI/BMT groups. We focused on the longer lasting ACE inhibitor enalapril, since patient compliance is considerably higher with single daily drug dosing than with the multiple daily dosing necessary with captopril. To our knowledge these results are the first examples of mitigation with the same drug for multiple organ injuries in the same animals after a single high dose of TBI. Such a mitigator would be relevant to survivors of acute hematological and gastrointestinal radiation syndromes following a nuclear accident or radiological terrorism event in which some bone marrow may be spared (e.g. by partial shielding or mitigated by supportive care or BMT).
MATERIALS AND METHODS
Animal Welfare
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical College of Wisconsin, Milwaukee, WI. Female rats (WAG/RijCMCR) were housed in a moderate security barrier. Following IACUC directives, the rats were considered morbid and euthanized if they met specific veterinarian's criteria that included at least three of the following: 1. greater than 20% loss in body weight; 2. inactivity on two consecutive days, defined as no movement unless actively stimulated; 3. lack of grooming that became worse after 24 h; 4. breathing rates of less than 60 or greater than 250 breaths per minute; and 5. hunched posture, death pose on 2 consecutive days. Necropsies were performed on some rats to examine the cause of the morbidity.
Data from rats were censored if they were lost for reasons unrelated to radiation-induced injury such as anesthesia when drawing blood for BUN measurements or if they were later sacrificed for unrelated studies that used the same model.
Dosimetry for Total Body Irradiations with Bone Marrow Transplant
For TBI, unanesthetized 8- to 10-week-old rats weighing approximately 140 g were immobilized in a plastic jig and irradiated using a XRAD 320KV orthovoltage X-ray system. The heads were shielded to facilitate food intake. The X-ray system was operated at 320 kVp and 13 mAs with a half value layer of 1.4 mm Cu. During irradiation, each rat was confined in a separate chamber in a plastic jig, which allows exposure of four rats simultaneously. The four chambers were placed on a plane perpendicular to the beam direction with distance from source to the midline of rats to be set at 33.5 cm. Collimator jaws were used to define a radiation field to be 22.5 × 22.5 cm2 at midline and large enough to cover all four chambers with adequate (at least 2 cm) margin.
A soft X-ray ionization chamber (PTW, Freiburg, Germany) was used to collect depth dose information. Absolute calibration measurements were made using a Farmer-type ionization chamber and a DOSE-1 electrometer (IBA Dosimetry, Schwarzenbruck, Germany). This system was calibrated for the orthovoltage energy range at the Accredited Dosimetry Calibration Laboratory located at University of Wisconsin (Madison, WI). Measurements performed in this laboratory are directly traceable to the National Institute of Standards and Technology. The ionization was measured in air and then converted to absolute dose in water following the American Association of Physicists in Medicine Task Group-61 protocol (11). The dose rate for TBI was defined at the midline of the rat and was calculated to be 1.73 Gy/min using measured output of the machine and the depth dose data. The irradiation time, including appropriate timer error of the X-ray machine, was then calculated to deliver 11, 11.25 or 11.5 Gy prescription doses in one fraction as given previously (8, 9, 12). The total prescription dose was delivered using one posterior-to-anterior beam. The dose fall-off from the surface to the midline of the rat was 17%. Gafchromict film EBT2 (International Specialty Products Inc., Wayne, NJ) sandwiched between solid water phantom slabs was used to obtain profile distributions. The dose at the centers of the four rat chambers varied by 2%, and rats were randomly assigned to chambers to avoid any resulting bias. All irradiated rats received a syngeneic bone marrow transplant a few hours after irradiation (8, 9, 12) to prevent acute hematopoietic injury. The irradiated rats were randomized for treatment with ACE inhibitors as described in each study and/or followed until all concurrent, age-matched, nondrug-treated controls were euthanized due to morbidity.
Drug Delivery
Animals were treated as previously described (13) with captopril (Sigma-Aldrich LLC, St. Louis, MO), enalapril (gift from Merck & Co. Inc., Rahway, NJ or Sigma-Aldrich LLC) or fosinopril (Sequoia Research Products Ltd., Pangbourne, UK) added to the drinking water. Doses were comparable to those in the clinic on a mg/m2/day basis and previously demonstrated mitigation of radiation-induced injury to the lung (13–15). Captopril was provided to rats at a concentration of either 150 or 300 mg/L, with a mean effective dose of 88 or 176 mg/m2/day, respectively. Enalapril was provided at 30, 40 or 60 mg/L for average doses of 18, 24 or 36 mg/m2/day, respectively. Fosinopril was provided at 100 mg/L, making the effective dose 60 mg/m2/day. The drugs were started one week after irradiation and continued until study termination. Animals were weighed at 42 and 84 days at the start and end of pneumonitis (Table 1). Numbers of irradiated rats in each group were: 11 Gy = 48; 11.25 Gy = 12; 11.5 Gy = 33; 12 Gy = 32; 11 Gy + captopril 88 mg/m2/day = 10; 11 Gy + captopril 176 mg/m2/day = 19; 11 Gy + enalapril 36 mg/m2/day = 5; 11 Gy + fosinopril mg/m2/day = 11; 11.25 Gy + enalapril 24 mg/m2/day = 10; 11.5 Gy + captopril 176 mg/m2/day = 12; and 11.5 Gy + enalapril 176 mg/m2/day = 12. Some of the rats in the control (unirradiated) and 11 Gy TBI groups were also part of other ongoing experiments including those reported in a previously published article (8). Fewer rats were randomized to drug treated groups based on previous studies in which the drugs reduced morbidity (13).
TABLE 1.
Summary of Body Weight in Grams (wt) in Surviving Rats after 11 Gy TBI/BMT with Drugs
| 42 days |
84 days |
|||
|---|---|---|---|---|
| Group | Mean wt (percentage of starting wt) | n | Mean wt (percentage of starting wt) | n |
| 11 Gy | 107 | 24 | 109 | 17 |
| 11 Gy + captopril 176 mg/m2/day | 112* | 11 | 118 | 8 |
| 11 Gy + captopril 88 mg/m2/day | 105 | 5 | 113 | 2 |
| 11 Gy + enalapril 36 mg/m2/day | 111 | 5 | 122* | 4 |
| 11 Gy + fosinopril 60 mg/m2/day | 111 | 11 | 112 | 8 |
P < 0.05 vs. 11 Gy (no drug).
Breathing Interval Measurements
The breathing rates and body weights were measured as described previously (16) in randomly selected rats from each group from 4–12 weeks after irradiation. In brief, rats were restrained in a Plexiglas jig and placed in an airtight box connected to a differential pressure transducer. The mean breathing rate in each rat was calculated from a minimum of four steady regions of recording lasting at least 15 s each. The reciprocal of the mean breathing rate (breathing interval) (14) for each rat was used for statistical analysis using median with 25–75% ranges.
Pulmonary Vascular Resistance and Terminal Arteriole Count
Pulmonary vascular resistance and terminal arteriole count were measured as described previously (8, 17). Briefly, the lungs and heart were harvested en bloc and perfused with 5% bovine serum albumin in physiological saline solution (Equitech-Bio Inc., Kerrville, TX). The pulmonary artery pressure was measured at 30, 20, 15, 10 and 5 ml/ min. The flow was then stopped and the closing pressure was recorded. The flow rates were normalized by the rat body weight and the pressure versus flow data were fit to a simple pulmonary vascular distensibility model (17) to determine pulmonary vascular resistance at a flow rate of 100 ml/min/kg.
To compare arterial densities 1-bromo-perfluoro-octane was added as perfusate for X-ray contrast. The airway pressure was maintained at 6 mm Hg. The intravascular pressure was set to 30 mm Hg, after conditioning by pressure cycling from 0–35 mm Hg several times. High-magnification microfocal angiographic images of isolated lungs were acquired. Applying a global threshold and a seeded region growing algorithm, the 3D isotropic CT data was used to determine the number of terminal vessels in the arterial tree, expressed as the “terminal arteriole count”.
Lung Histology
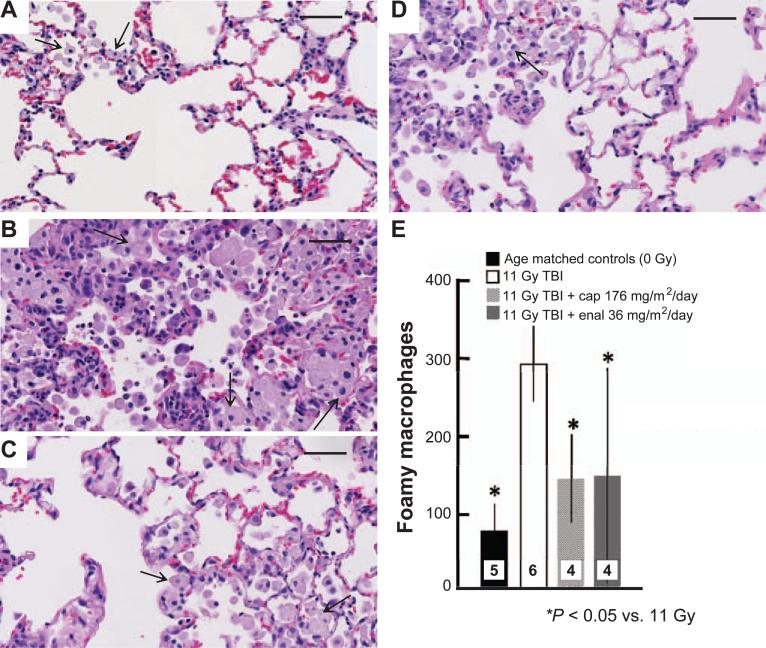
After perfusion and imaging, the inflated lungs from TBI-treated rats were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburg, PA), and the left lung was embedded in paraffin (8). Whole mount sections were cut (4 μm), processed and stained with hematoxylin and eosin (H&E) (Richard-Allan Scientifice, Kalamazoo, MI) and scanned at high resolution by the CRI Pediatric Biobank and Analytical Tissue Core at the Medical College of Wisconsin. Two operators blinded to the source of the lung sections counted foamy macrophages in 5 random high power fields in corresponding regions of each lung. The macrophages/lung section were totaled and averaged for statistical analysis.
Measuring Blood Urea Nitrogen: A Surrogate Marker for Radiation Nephropathy
Rats were anesthetized with 3–5% isoflurane for blood draws by retro-orbital bleeds conducted by an experienced technician. At 70 days three rats were lost under anesthesia and eliminated (censored) from the study [11.5 Gy + enalapril 18 mg/m2/day (2) and 11.5 Gy + captopril 176 mg/m2/day (1)]. The BUN was assayed from serum as described previously (19) using a urease-nitroprusside colorimetric assay. BUN values were expressed as mg/dl of serum and median with 25–75% ranges were plotted and used for statistical analysis. Irradiated rats with BUN >120 mg/dl were previously confirmed to have renal damage (18).
Statistical Analyses
The morbidity/mortality (survival as approved by the IACUC criteria) of rats after treatments was evaluated by a Kaplan-Meier survival plot and expressed as percent morbidity. Significance was analyzed by the Peto-Peto Wilcoxon test. Body weight was analyzed by one-way analysis of variance (ANOVA) followed by multiple comparisons versus the irradiated alone (without drug) group by Dunnett's method.
The breathing interval was used as a measure of lung function with time after irradiation. Higher breathing rates and lower breathing intervals have been associated with lung damage (14). As used previously to account for attrition, we set the breathing interval to 0 for animals that died during pneumonitis before the data point under consideration (14). Results were analyzed by the Kruskal-Wallis ANOVA on ranks. Median breathing intervals with 25–75% ranges were calculated. All pairwise multiple comparisons were tested by Dunn's test. A difference of ranks was considered significant if P < 0.05. Pulmonary vascular resistance, terminal arteriole count and foamy macrophage count were used to confirm pneumonitis after irradiation. Results were analyzed by ANOVA, all pairwise multiple comparisons were performed using the Holm-Sidak method.
To measure kidney function, median BUN values with 25–75% ranges were calculated. Animals with BUN >120 mg/dl predicted renal failure and were euthanized by direction of the IACUC (10). These rats were assigned a BUN of 300 mg/dl to account for attrition at their next scheduled time point. Results of BUN values were analyzed by Mann-Whitney rank sum tests to determine significant differences between groups.
RESULTS
Radiation-Dose Response after TBI/BMT
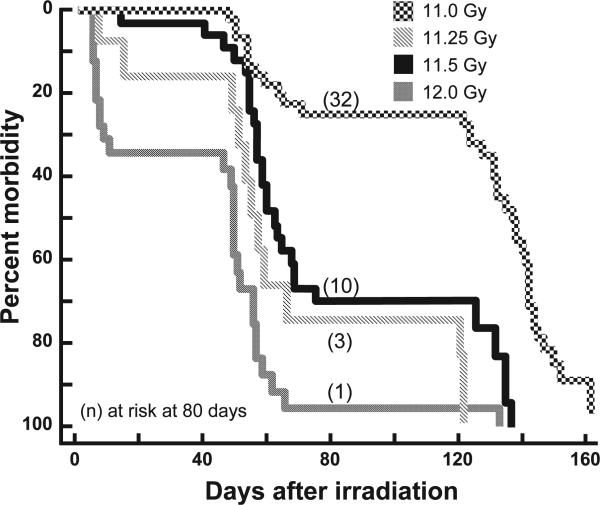
Doses of 11, 11.25, 11.5 and 12 Gy were used for TBI/ BMT to develop models for our mitigation studies (Fig. 1). All doses yielded two phases of morbidity, the first between 40–80 days and the second after 120 days. In previous studies these phases corresponded to radiation pneumonitis (8, 13, 16) and radiation nephropathy (19), respectively. After exposure to 11 Gy in the current study, ~25% of the rats were lost between 40–80 days and all surviving rats were euthanized by 160 days. Doses of 11.25 or 11.5 Gy had a similar survival profile except a higher number (70%) of the animals were morbid at times corresponding to pneumonitis while the remainder were euthanized at the time corresponding to nephropathy by 140 days in previous studies. The highest dose (12 Gy TBI/BMT) resulted in a loss of >35% of the rats before 40 days, at times coinciding with acute radiation-induced GI toxicity. Over 90% of the remaining animals were lost by the end of the first phase. This dose was therefore not suitable to study mitigation of late effects, since there were too few survivors for evaluation of the later nephropathy that occurs after 80 days after exposure. Instead we used 11, 11.25 and 11.5 Gy TBI/BMT doses to test the efficacy of ACE inhibitors to mitigate the late effects of radiation pneumonitis and nephropathy (see sections below).
FIG. 1.
Dose response to total body irradiation. Kaplan-Meier plots for morbidity are shown after increasing doses of total body irradiation at 11, 11.25, 11.5 and 12 Gy followed by bone marrow transplants (TBI/BMT). Numbers of rats at risk at 80 days after irradiation are shown in parenthesis. Rats at risk (n) at 45 days after TBI (i.e., the start of pneumonitis) were as follows: 11 Gy = 45, 11.25 Gy = 10, 11.5 Gy = 31 and 12 Gy = 16. pneumonitis Survivors of at 80 days were ultimately lost due to radiation nephropathy at all doses. Incidences for some of the unirradiated and 11 Gy TBI rats are also part of a previously published article (8).
Mitigation by ACE Inhibitors after 11 Gy TBI/BMT
Survival
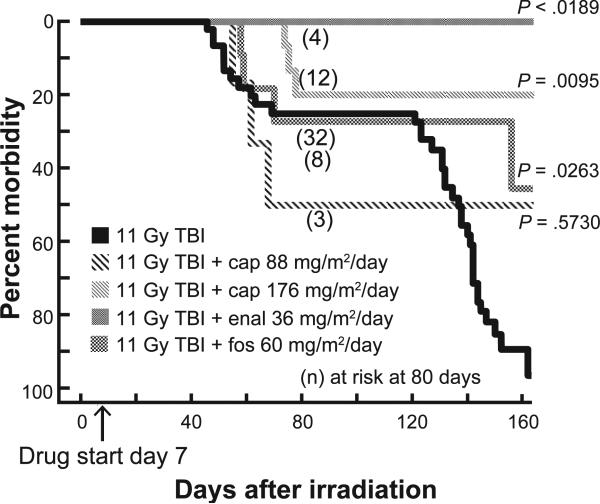
Only rats that survived to 35 days and were then at risk for pneumonitis and nephropathy were included to study mitigation. Previous studies have demonstrated that acute radiation effects such as gastrointestinal toxicity or bone marrow failure that occur in the first month after irradiation are not well mitigated by ACE inhibitors (20, 21). In the first study we tested the effects of three ACE inhibitors after 11 Gy TBI/BMT (Fig. 2). Captopril was used at two doses (88 and 176 mg/m2/day). The lower dose did not improve survival but the higher dose of captopril did (P = 0.001). Irradiated animals given the higher dose of captopril had a higher body weight after 42 days but not after 84 days [compared to those not treated with the drug (Table 1)]. Enalapril (36 mg/m2/day) but not fosinopril (60 mg/m2/day) appeared to improve survival through the pneumonitis phase at 80 days. Fosinopril also did not demonstrate efficacy against radiation pneumonitis after 13 Gy irradiation to the whole thorax only (13). Rats given enalapril in the current study had higher body weight after 84 days compared to those that were not treated with enalapril.
FIG. 2.
Mitigation by ACE inhibitors after 11 Gy TBI. Kaplan-Meier plots for morbidity show effects of ACE inhibitors for mitigation of multiple organ dysfunction. Drugs were started 7 days after irradiation (marked by an arrow on the X axis) and continued. Rats at risk (n) at 45 days after TBI (i.e., the start of pneumonitis) were as follows: 11 Gy alone = 45; 11 Gy + captopril (88 mg/m2/day) = 6; 11 Gy = captopril (176mg/m2/day)15; 11 Gy + enalapril (36 mg/m2/day) = 5; and 11 Gy + fosinopril (60 m/m2/day) = 11. Numbers of rats at risk at 80 days after irradiation are shown in parenthesis. Captopril (88 mg/m2/day) and fosinopril did not appear to be effective against pneumonitis while captopril (176 mg/m2/day), enalapril (36 mg/m2/day) and fosinopril (60 mg/m2/day) reduced morbidity at 160 days (P < 0.05 by Peto-Peto Wilcoxon tests). Incidences for some of the unirradiated and 11 Gy TBI rats are also part of a previously published article (8).
Pneumonitis
Breathing rates have been used to measure lung function and they are increased in irradiated rodents during pneumonitis (14, 22–24). We measured breathing rates as described previously (14) and used the corresponding breathing intervals (reciprocal of breathing rate) for analysis. The median (25–75% ranges) breathing intervals of rats exposed to 1 Gy TBI/BMT at four time points are shown in Table 2. Irradiated animals not treated with drugs demonstrated a drop in breathing intervals that were lowest at 42 days but then increased in surviving rats at 56 and 70 days. Rats given ACE inhibitors showed a delay in the timing of the lowest breathing interval, which occurred at day 56 versus day 42 in irradiated rats without drug (Table 2). The median breathing interval increased after 70 days in all groups except those given the low dose of captopril (88 mg/m2/day). There was a 50% loss of rats in the group receiving low captopril dose due to pneumonitis, resulting in a median breathing interval of 0 for these animals (Fig. 2). Based on these results we chose to follow animals treated with lower doses of enalapril in future studies, since no rats on that drug were lost while all rats in the 11 Gy TBI/BMT group fit the criteria for euthanization. We also tested the high dose of captopril for mitigation, in combination with higher doses of 11.25 and 11.5 Gy TBI/BMT.
TABLE 2.
Summary of Breathing Interval after 11 Gy TBI/BMT with Drugs
| Days after irradiation | 11 Gy TBI + drug (mg/m2/day) | Median | 25% | 75% | n | P < 0.05 vs. no drug at time |
|---|---|---|---|---|---|---|
| 28 | no drug | 0.462 | 0.416 | 0.503 | 24 | n/a |
| captopril 176 | 0.466 | 0.447 | 0.486 | 11 | no | |
| captopril 88 | 0.495 | 0.466 | 0.504 | 5 | no | |
| enalapril 36 | 0.487 | 0.456 | 0.528 | 5 | no | |
| fosinopril 60 | 0.487 | 0.467 | 0.527 | 11 | no | |
| 42 | no drug | 0.337 | 0.259 | 0.419 | 24 | n/a |
| captopril 176 | 0.466 | 0.419 | 0.527 | 11 | yes | |
| captopril 88 | 0.511 | 0.451 | 0.524 | 5 | yes | |
| enalapril 36 | 0.446 | 0.405 | 0.454 | 5 | no | |
| fosinopril 60 | 0.433 | 0.312 | 0.444 | 11 | no | |
| 56 | no drug | 0.376 | 0.276 | 0.425 | 24 | n/a |
| captopril 176 | 0.306 | 0.289 | 0.393 | 11 | no | |
| captopril 88 | 0.256 | 0.171 | 0.398 | 5 | no | |
| enalapril 36 | 0.262 | 0.254 | 0.339 | 5 | no | |
| fosinopril 60 | 0.325 | 0.295 | 0.414 | 11 | no | |
| 70 | no drug | 0.411 | 0 | 0.455 | 24 | n/a |
| captopril 176 | 0.335 | 0.262 | 0.374 | 11 | no | |
| captopril 88 | 0 | 0 | 0.434 | 5 | no | |
| enalapril 36 | 0.355 | 0.211 | 0.398 | 5 | no | |
| fosinopril 60 | 0.376 | 0.0503 | 0.457 | 11 | no |
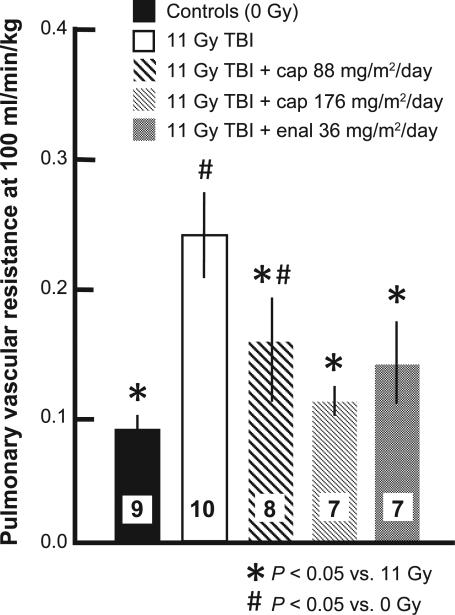
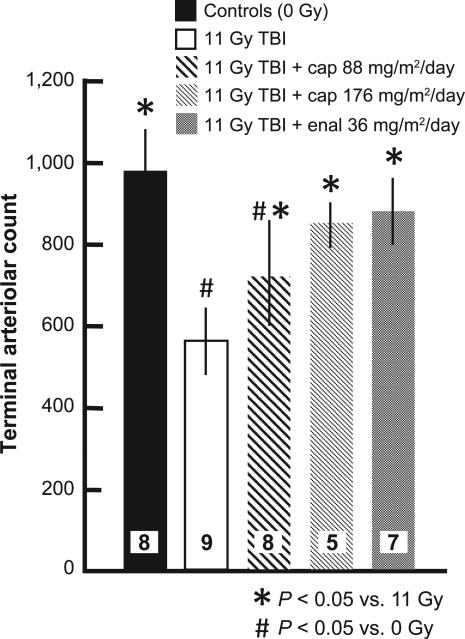
Some groups of rat lungs were also evaluated by invasive assays during pneumonitis (between 42 and 60 days), with isolated perfused lungs ex vivo (17). The pulmonary vascular resistance of these lungs was markedly increased after 11 Gy irradiation (Fig. 3) and this effect was mitigated by both the higher and lower dose of captopril as well as the higher dose of enalapril. The decrease in pulmonary arteriolar density after 11 Gy irradiation, which was evaluated by high-density angiography as described in (17), was also mitigated by captopril and enalapril (Fig. 4). Finally, histological sections were examined and foamy macrophages infiltrating the lungs were estimated (Fig. 5A–E). The number of macrophages was lower in irradiated lungs of rats given the higher dose of captopril or enalapril (Fig. 5E) than those irradiated and not treated with ACE inhibitors. Taken together, secondary end points such as the increase in breathing rate, vascular resistance and infiltrating macrophages as well as decrease in arteriolar density identify pneumonitis during the first phase of morbidity in Fig. 2. Mitigation of the response in these end points support mitigation of radiation pneumonitis by ACE inhibitors at certain doses.
FIG. 3.
Increase in pulmonary vascular resistance and mitigation by captopril and enalapril after 11 Gy TBI/BMT. Rats were irradiated and treated with two doses of captopril (88 and 176 mg/m2/day) or one dose of enalapril (36 mg/m2/day) starting 7 days after irradiation. Pulmonary vascular resistance was measured at 56 days after irradiation. Values are shown as mean ± 95% confidence intervals. Numbers in bars show the number of rats per group. *P < 0.05 vs. 11 Gy TBI; #P < 0.05 vs. 0 Gy. Values for unirradiated and 11 Gy TBI rats are the same as a previously published article (8).
FIG. 4.
Loss of pulmonary vessels and mitigation by captopril and enalapril after 11 Gy TBI/BMT. Rats were irradiated and treated with two doses of captopril (88 and 176 mg/m2/day) or one dose of enalapril (36 mg/m2/day) starting 7 days after irradiation. The terminal arteriolar counts for each group were obtained (see Materials and Methods for details) and are shown as mean ± 95% confidence intervals. Numbers in bars show the number of rats per group. *P < 0.05 vs. 11 Gy TBI; #P < 0.05 vs. 0 Gy. Values for unirradiated and 11 Gy TBI rats are the same as a previously published article (8).
FIG. 5.
Histology showing H&E stained lung sections from irradiated rats (11 Gy TBI/BMT between 50–60 days) and those treated with a high dose of captopril (176 mg/m2/day) and enalapril (36 mg/m2/day). Panel A: Unirradiated lung; panel B: 11 Gy; panel C: 11 Gy + captopril; panel D: 11 Gy + enalapril. Arrows point to foamy macrophages. Also note increased cellularity and alveolar thickening in irradiated lungs. Bar = 50 μm. Panel E: Graph showing foamy macrophage counts in H&E stained lung sections. Results show a decrease in the number of foamy macrophages in lungs of rats treated with the ACE inhibitors. Numbers in bars show the number of rats per group. *P < 0.05 vs. 11 Gy.
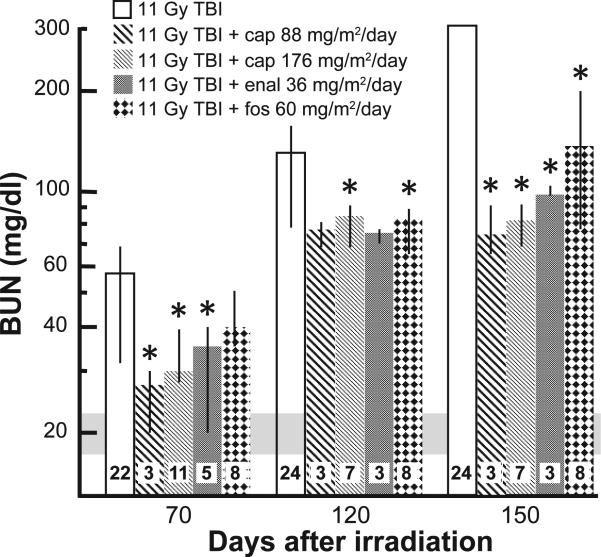
Nephropathy. Progression of radiation nephropathy was followed by measuring the BUN (azotemia) at 70, 120 and 150 days after irradiation (Fig. 6). The median BUN (25–75% ranges) was lower for rats given the higher dose of captopril at all three times after TBI/BMT compared to rats that were only irradiated (Fig. 6). All irradiated and drug-treated rats had lower BUNs at 150 days when compared to rats given only 11 Gy TBI/BMT (Fig. 6) and most of the rats without drug treatment were morbid (Fig. 2).
FIG. 6.
Time course of increase in BUN after 11 Gy TBI and mitigation by ACE inhibitors. Results of median BUN values with 25–75% ranges are shown for 70, 120 and 150 days, respectively, after 11 Gy TBI/BMT. The numbers in each bar show the number of rats evaluated in the group (n). The horizontal gray bar show values of BUN in unirradiated rats (controls). All treatments with ACE inhibitors mitigated azotemia at 150 days after irradiation. *P < 0.05 vs. 11 Gy alone at the corresponding time.
Mitigation by ACE Inhibitor Enalapril after 11.25 Gy TBI/BMT
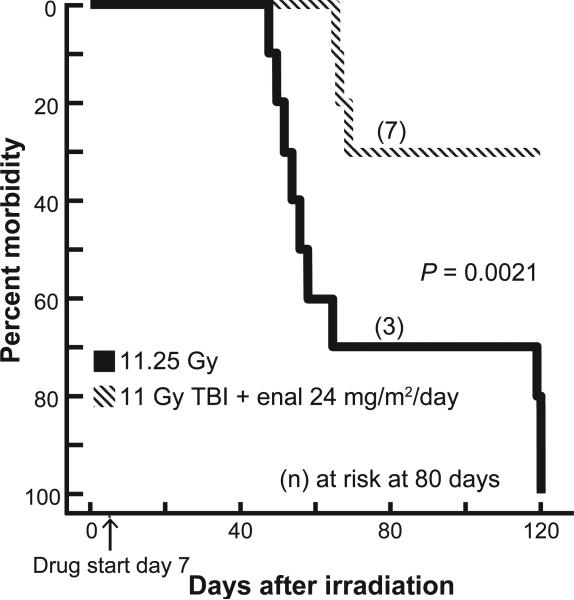
Survival
Based on the promising results of our study of exposure with 11 Gy, we tested a lower dose of the drug enalapril (24 mg/m2/day) for mitigation with an exposure of 11.25 Gy. Survival was improved by enalapril (24 mg/m2/day) at 120 days after 11.25 Gy TBI/BMT (P = 0.002) (Fig. 7).
FIG. 7.
Mitigation by ACE inhibitors after 11.25 Gy TBI. Kaplan-Meier plots for morbidity show effects of enalapril for mitigation of multiple organ dysfunction. Enalapril (24 mg/m2/day) was started 7 days after irradiation (marked by an arrow on the X axis) and continued. Rats at risk (n) at 45 days after TBI (i.e., the start of pneumonitis) were as follows: 11.25 Gy = 10 and 11 Gy + enalapril (24 mg/m2/day) = 10. Enalapril reduced the morbidity for both phases, radiation pneumonitis and nephropathy.
Mitigation by ACE Inhibitors Enalapril and Captopril after 11.5 Gy TBI/BMT
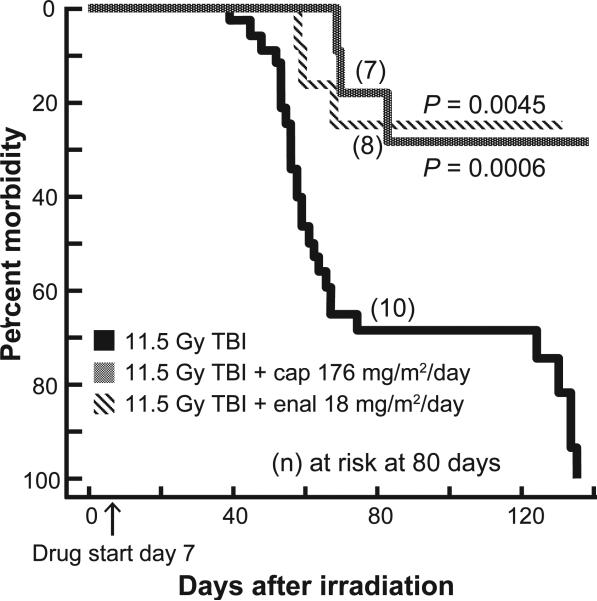
Survival
Since enalapril at 24 mg/m2/day mitigated morbidity after 11.25 Gy irradiation, we tested an even lower dose of the drug (18 mg/m2/day) after exposure to 11.5 Gy. Captopril was also used at the dose (176 mg/m2/day) that had been optimized in a previous study (25). Both captopril (176 mg/m2/day) and enalapril (18 mg/m2/day) improved survival of rats after 11.5 Gy TBI/BMT by 140 days (Fig. 8).
FIG. 8.
Mitigation by ACE inhibitors after 11.5 Gy TBI. Kaplan-Meier plots for morbidity show effects of ACE inhibitors for mitigation of multiple organ dysfunction. Drugs were started 7 days after irradiation (marked by an arrow on the X axis) and continued. Rats at risk (n) at 45 days after TBI (i.e. the start of pneumonitis) were as follows: 11.5 Gy = 31; 11.5 Gy + captopril (176 mg/m2/day) = 11; and Gy + enalapril (18 mg/m2/day) = 12. Captopril (176 mg/m2/day) and enalapril (18 mg/m2/day) reduced morbidity at both phases of the survival curves as determined by Peto-Peto Wilcoxon tests.
Pneumonitis
The median breathing intervals after 11.5 Gy TBI/BMT are shown in Table 3. Without any drugs this value dropped and continued to fall until day 70, at which time 70% of the rats had died due to pneumonitis (Fig. 8). Captopril improved the breathing interval at 42, 56 and 70 days compared to the rats that had received TBI/BMT only. In contrast enalapril (18 mg/m2/day) did not significantly improve the breathing interval (Table 3), and that group's lowest median breathing interval was at day 56 after 11.5 Gy TBI/BMT.
TABLE 3.
Summary of Breathing Interval after 11.5 Gy TBI/BMT with Drugs
| Days after irradiation | 11.5 Gy TBI + drug (mg/m2/day) | Median | 25% | 75% | n | P < 0.05 vs. no drug at time |
|---|---|---|---|---|---|---|
| 28 | no drug | 0.495 | 0.444 | 0.522 | 29 | n/a |
| captopril 176 | 0.52 | 0.452 | 0.567 | 14 | no | |
| enalapril 18 | 0.516 | 0.474 | 0.553 | 12 | no | |
| 42 | no drug | 0.332 | 0.274 | 0.462 | 29 | n/a |
| captopril 176 | 0.459 | 0.422 | 0.488 | 14 | yes | |
| enalapril 18 | 0.371 | 0.347 | 0.425 | 12 | no | |
| 56 | no drug | 0.264 | 0 | 0.325 | 29 | n/a |
| captopril 176 | 0.395 | 0.309 | 0.439 | 14 | yes | |
| enalapril 18 | 0.279 | 0.243 | 0.348 | 12 | no | |
| 70 | no drug | 0 | 0 | 0.36 | 29 | n/a |
| captopril 176 | 0.353 | 0.289 | 0.405 | 14 | yes | |
| enalapril 18 | 0.304 | 0 | 0.392 | 12 | no |
On inspection (necropsy) of rats that were morbid between 40–80 days after >11 Gy TBI/BMT (with or without ACE inhibitor treatment) all rats had grossly abnormal lungs (n = 12). The pleural surfaces were pale. Some lungs were mottled with irregular dark patches and/or had raised, opaque foci that were white in color. The lungs were also larger in appearance than normal and did not spontaneously deflate. In addition, 7/12 (58%) rats had pleural effusions.
Nephropathy
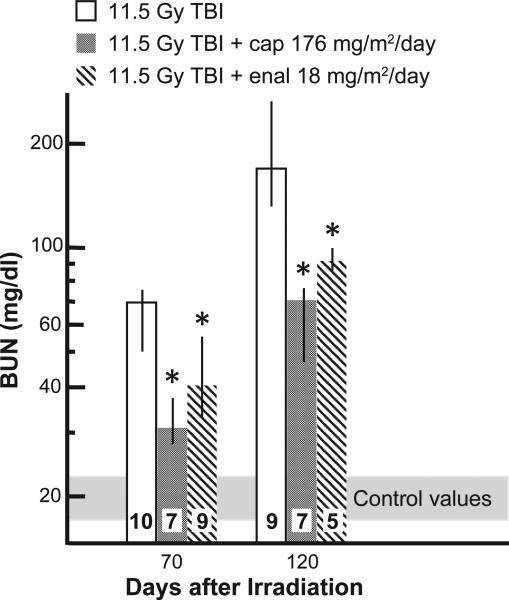
The BUNs were lower in rats given the higher dose of captopril (176 mg/m2/day) and lower dose of enalapril (18 mg/m2/day) at 70 days as well as 120 days after 11.5 Gy TBI/BMT (Fig. 9).
FIG. 9.
Time course of increase in BUN after 11.5 Gy TBI and mitigation by ACE inhibitors. Results of median BUN values with 25–75% ranges are shown for 70 and 120 days after 11 Gy TBI. All treatments with ACE inhibitors mitigated azotemia at 70 and 120 days after irradiation. The numbers in each bar show the number of rats evaluated in the group (n). *P < 0.05 vs. 11.5 Gy alone at the corresponding time.
DISCUSSION
In this article, we described responses after TBI/BMT in rats with graded morbidity, and demonstrated mitigation of injuries to the lungs and kidneys in the same animals using an ACE inhibitor. Our primary end point was survival. Captopril (176 mg/m2/day) or enalapril (18–36 mg/m2/day) improved survival past 120 days after 11–11.5 Gy TBI/ BMT. In this and previous studies, acute lung injury (pneumonitis) in this model occurred between 40–80 days after TBI (8) while radiation nephropathy was lethal at later times [beyond 90 days (9)]. In this current study, pneumonitis was monitored by a reversible increase in breathing rate (breaths/min) and thus a corresponding decrease in breathing intervals (Table 2), which was confirmed by vascular resistance measurement (Fig. 3) and arteriolar densities ex vivo (Fig. 4) as well as by histopathology of the lungs (Fig. 5A–E). With 12 Gy TBI/ BMT, greater than 90% of the rats were morbid from pneumonitis whereas 25–75% rats survived doses of TBI/ BMT from 11–11.5 Gy. Pleural and pericardial effusions were observed in some rats and may have also contributed to respiratory failure. Rats treated with the ACE inhibitor captopril had higher median breathing intervals at the peak of pneumonitis (42 days) compared to those not treated with the ACE inhibitors. All rats surviving pneumonitis eventually died due to radiation nephropathy, which was quantified as we have described previously (18) by a continuous rise in BUN beginning 80 days after TBI. ACE inhibitors captopril and enalapril slowed the rise in BUN and improved survival through nephropathy. Though no rats would survive radiation nephropathy indefinitely, we observed that ACE inhibitors can increase survival by >6 months, which is approximately 25% of the life span of our rats (results not shown). Other organ injuries were not assessed, but the surviving rats exhibited good activity levels and grooming, adequate food and water intake and healthy coats at termination. Body weights were acquired as an index of overall health and quality of life in irradiated rats. After recovery from radiation pneumonitis at 84 days with 11 Gy TBI/BMT, rats that were given enalapril had greater increase in body weight than untreated cohorts. The changes in morbidity after 84 days (i.e., after pneumonitis) did not appear to be closely related to changes in body weight, suggesting that the rats did not become morbid due to lack of appetite or ability to absorb nutrition during radiation nephropathy.
In addition to breathing rates, we have previously reported a number of other parameters that confirm lung injury and pneumonitis 42 days after 11 Gy TBI/BMT. The vascular resistance, arteriole density, ACE activity as well as histopathology measuring vascular thickening and macrophage infiltration were all higher in 11 Gy irradiated lungs compared to unirradiated controls (8). Vascular distensibility was also decreased in ex vivo lungs 42 days after 11 Gy TBI at which time right ventricular hypertrophy was present (results not shown). Vascular distensibility is the passive ability of blood vessels to increase in diameter with increases in pressure and is a reflection of vessel stiffening (17).
Radiation nephropathy has been well characterized in rats given TBI/BMT with lower doses (up to 10 Gy) in a similar model (9, 10). Indeed, the response of male rats after a single dose of TBI/BMT (9, 10) is reported, but only fractionated doses have been tested in female rats (12, 18, 26). The current study describes progression of radiation nephropathy and mitigation by ACE inhibitors in female rats given unfractionated TBI. Taken together with other rat studies (7, 9, 10), the kidney is the most sensitive organ to radiation even if bone marrow toxicity is spared by either a bone marrow transplant or at least 5% shielding of the marrow. However, although radiation nephropathy can diminish quality of life, dialysis and transplant are potential treatment options. Failure of other organ systems such as the gut (7, 27), heart (5, 28) or brain usually occur after higher doses of radiation (6).
Injury of kidneys and lungs is common in patients who have received total body irradiation in preparation for bone marrow transplant (1–3), supporting the clinical relevance of the rat model employed in this study. Interest in mitigation of radiation injuries, which increased in the years following the 9–11 attacks, has prompted an increase in funding to develop countermeasures. A number of agents have been investigated with funding from the NIAID and the Biomedical Advanced Research and Development Authority (BARDA) to mitigate radiation pneumonitis, a topic that has been recently reviewed (29). Briefly, in addition to ACE inhibitors, other promising drugs are currently under investigation, which include antioxidants such as two classes of superoxide dismutase mimetics (8, 24, 30–32) and the tyrosine kinase inhibitor genistein. Statins (33), oxidized glutathione variants (34) and nutraceuticals such as triptolide and flaxseed lignin (35, 36) have also been tested. Steroids have demonstrated potential to mitigate lung injury in irradiated animals (37–40). Survival of mice treated with prednisolone 80 days after 2,800 rads in two fractions was better in mice that were irradiated only, but the survival rate dropped sharply when the treatment was discontinued (38). Of these drugs, genistein and EUK compounds mitigated injury to more than one organ (8, 31, 41, 42).
Radiation nephropathy in rodents has been mitigated by ACE inhibitors as well as angiotensin receptor blockers (9, 43, 44). A comprehensive review of these and other agents has been described (43) but not specifically for a mitigating regimen relevant to the NIAID Radiation and Nuclear Countermeasures Program. Dexamethasone has been shown to increase survival time and ameliorate radiation nephropathy in rats (45–47). Interestingly, treatment with both dexamethasone and captopril produced greater delay in the progression of renal injury than either agent alone (47). Chronic administration of acetylsalicylic acid (aspirin) was also effective (48). At this time we are testing the effect of Neupogent® and antibiotics given 2 weeks after TBI without head shielding and in combination with ACE inhibitors, parameters that are prioritized in the NIAID/BARDA initiative.
In September 2012 the U.S. Food and Drug Administration (FDA) recommended that it would be beneficial if preclinical development of countermeasures for radiation injuries were conducted in parallel with studies to decrease radiation injuries in cancer patients (49). This is because it is not ethical to conduct clinical trials with total body doses and schedules of radiation that would be relevant to a nuclear attack or accident. Very few countermeasures mentioned above have demonstrated efficacy in irradiated humans in trials on cancer patients receiving radiotherapy. Wang et al. (50) reported that incidental use of ACE inhibitors had no effect on radiation pneumonitis. In contrast, Bezjak et al. (51) showed that similar treatment resulted in a decrease in the incidence of pneumonitis in irradiated lung cancer patients, from 29% (34/118) to 12.5% (3/24). A similar analysis found that incidental use of ACE inhibitors cuts pneumonitis occurrence in irradiated lung cancer patients from 11% (11/101) to 2% (1/61) (3). A meta-analysis of these three studies gives a pooled odds ratio of 0.48 with P = 0.046 for subjects using ACE inhibitors compared to no drug, pointing out that ACE inhibitors reduce the risk of clinical pneumonitis. Jenkins et al. (52, 53) also observed decreased pneumonitis in patients taking ACE inhibitors. The more widespread use of newer more efficacious ACE inhibitors in the later studies may explain the increased efficacy of the ACE inhibitors. We also tested captopril versus placebo in a prospective trial to mitigate chronic renal failure and after radiation-based hematopoietic stem cell transplant (TBI/HSCT) (1, 2). Survival as well as nephropathy and pulmonary-related mortality were improved in patients treated with captopril (1–3). As well as supporting the FDA recommendation and indicating the potential for reduced radiation injury, these clinical studies support the safety of ACE inhibitor use in irradiated human subjects. In addition, we and others have demonstrated ACE inhibitors to mitigate radiation injuries in preclinical studies of lung (25, 54, 55), kidney (9, 56), brain (57) and skin (58).
In summary, we have developed a model of radiation-induced multiple organ injury in female rats, in response to the NIAID countermeasures program for a terrorism-relevant radiological attack or nuclear accident. We delivered a high dose of 11–11.5 Gy unfractionated radiation in a whole animal model and provide new information regarding the characteristics and timing of morbidity after doses of TBI/BMT below the threshold for lethal gastrointestinal toxicity. Mitigation of the resulting pneumonitis and nephropathy with three ACE inhibitors was observed. One limitation of our model was the use of a bone marrow transplant to protect the rats from acute death by hematopoietic depletion, since such a procedure would not be practical after a mass casualty event. The highest doses of the ACE inhibitors captopril and enalapril that we tested were the most effective at mitigating the multiple organ injuries induced by TBI/BMT. Further studies in male, pediatric, juvenile and geriatric models are needed to develop ACE inhibitors for licensure and approval for use against radiation injuries.
ACKNOWLEDGMENTS
We thank Jayashree Narayanan for technical help and Merck Inc. for supplying enalapril. We are grateful to Dr. Natalya V. Morrow for the dosimetry and support with irradiation. The CRI Pediatric Biobank and Analytical Tissue Core at the Medical College of Wisconsin is supported by the MACC Fund. This work was funded by NIH/NIAID RC1 AI81294&S1, agreements U19 AI67734, 1R01AI101898 and U01-AI107305-01.
Footnotes
Mitigation is the use of a countermeasure to alleviate organ damage after injury but before manifestation of symptoms (59).
REFERENCES
- 1.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70:1546–51. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, et al. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83:292–6. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–43. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010;76:S108–15. doi: 10.1016/j.ijrobp.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 5.Ghobadi G, van der Veen S, Bartelds B, de Boer RA, Dickinson MG, de Jong JR, et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e639–46. doi: 10.1016/j.ijrobp.2012.07.2362. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ, Giaccia AJ. Clinical response of normal tissues. In: Radiobiology for the radiobiologist. 7th ed. Lippincott, Williams & Wilkins; Philadelphia: 2012. pp. 327–55. [Google Scholar]
- 7.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F, Fish BL, Szabo A, Doctrow SR, Kma L, Molthen RC, et al. Short-term treatment with a SOD/catalase mimetic, EUK-207, mitigates pneumonitis and fibrosis after single-dose total-body or whole-thoracic irradiation. Radiat Res. 2012;178:468–80. doi: 10.1667/RR2953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, et al. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat Res. 2009;171:164–72. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, et al. AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys. 2001;28:868–93. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 12.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys. 1989;16:1501–9. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 13.Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 2012;53:10–7. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Radiat Res. 2012;53:633–40. doi: 10.1093/jrr/rrs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PDR . Physician's desk reference. 65th ed. PDR Network LLC; Montvale: 2011. pp. 2394–7. [Google Scholar]
- 16.Zhang R, Ghosh SN, Zhu D, North PE, Fish BL, Morrow NV, et al. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84:487–97. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh SN, Wu Q, Mader M, Fish BL, Moulder JE, Jacobs ER, et al. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009;74:192–9. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors. Int J Radiat Oncol Biol Phys. 1993;27:93–9. doi: 10.1016/0360-3016(93)90425-u. [DOI] [PubMed] [Google Scholar]
- 19.Cohen EP, Moulder JE, Fish BL, Hill P. Prophylaxis of experimental bone marrow transplant nephropathy. J Lab Clin Med. 1994;124:371–80. [PubMed] [Google Scholar]
- 20.Moulder JE, Fish BL. Angiotensin converting enzyme inhibitor captopril does not prevent acute gastrointestinal radiation damage in the rat. Radiat Oncol Investig. 1997;5:50–3. doi: 10.1002/(SICI)1520-6823(1997)5:2<50::AID-ROI2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Moulder JE, Fish BL, Cohen EP, Klein JP, Davis, et al. “Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation”. Exp Hematol. 2011;39:521–2. doi: 10.1016/j.exphem.2011.02.006. Author reply 522–4. [DOI] [PubMed] [Google Scholar]
- 22.Travis EL, Meistrich ML, Finch-Neimeyer MV, Watkins TL, Kiss I. Late functional and biochemical changes in mouse lung after irradiation: Differential effects of WR-2721. Radiat Res. 1985;103:219–31. [PubMed] [Google Scholar]
- 23.Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res. 2010;173:10–20. doi: 10.1667/RR1911.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87:889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, et al. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75:1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulder JE, Holcenberg JS, Kamen BA, Cheng M, Fish BL. Renal irradiation and the pharmacology and toxicity of methotrexate and cisplatinum. Int J Radiat Oncol Biol Phys. 1986;12:1415–8. doi: 10.1016/0360-3016(86)90184-7. [DOI] [PubMed] [Google Scholar]
- 27.Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th ed. Lippincott, Williams & Wilkins; Philadelphia: 2012. Acute radiation syndrome. pp. 114–128. [Google Scholar]
- 28.Geist BJ, Lauk S, Bornhausen M, Trott KR. Physiologic consequences of local heart irradiation in rats. Int J Radiat Oncol Biol Phys. 1990;18:1107–13. doi: 10.1016/0360-3016(90)90446-q. [DOI] [PubMed] [Google Scholar]
- 29.DiCarlo AL, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, Williams JP. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiat Res. 2012;177:717–21. doi: 10.1667/rr2881.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas JS, Ye X, Leong KW, et al. Diverse functions of cationic mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radic Biol Med. 2011;51:1035–53. doi: 10.1016/j.freeradbiomed.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, et al. Salen mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11:359–72. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tovmasyan A, Sheng H, Weitner T, Arulpragasam A, Lu M, Warner DS, et al. Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators. Med Princ Pract. 2013;22:103–30. doi: 10.1159/000341715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161:560–7. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 34.Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert Opin Investig Drugs. 2008;17:1075–83. doi: 10.1517/13543784.17.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, et al. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Y, Yang S, Xiao Z, Wang W, Okunieff P, Zhang L. Triptolide alters mitochondrial functions. Adv Exp Med Biol. 2007;599:139–46. doi: 10.1007/978-0-387-71764-7_19. [DOI] [PubMed] [Google Scholar]
- 37.Berdjis CC, Brown RF. Histopathology of the effect of cortisone on the irradiated rat lung. Dis Chest. 1957;32:481–92. doi: 10.1378/chest.32.5.481. [DOI] [PubMed] [Google Scholar]
- 38.Gross NJ. Radiation pneumonitis in mice. Some effects of corticosteroids on mortality and pulmonary physiology. J Clin Invest. 1980;66:504–10. doi: 10.1172/JCI109881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross NJ, Narine KR, Wade R. Protective effect of corticosteroids on radiation pneumonitis in mice. Radiat Res. 1988;113:112–9. [PubMed] [Google Scholar]
- 40.Moss WT, Haddy FJ, Sweany SK. Some factors altering the severity of acute radiation pneumonitis: Variation with cortisone, heparin, and antibiotics. Radiology. 1960;75:50–4. doi: 10.1148/75.1.50. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: Effects in adolescent rats. Radiat Res. 2013;179:125–34. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill RP, Zaidi A, Mahmood J, Jelveh S. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011;101:73–9. doi: 10.1016/j.radonc.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulder JE, Robbins ME, Cohen EP, Hopewell JW, Ward WF. Pharmacologic modification of radiation-induced late normal tissue injury. Cancer Treat Res. 1998;93:129–51. doi: 10.1007/978-1-4615-5769-2_6. [DOI] [PubMed] [Google Scholar]
- 44.Robbins ME, Hopewell JW. Physiological factors effecting renal radiation tolerance: A guide to the treatment of late effects. Br J Cancer Suppl. 1986;7:265–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Geraci JP, Mariano MS, Jackson KL, Taylor DA, Still ER. Effects of dexamethasone on late radiation injury following partial-body and local organ exposures. Radiat Res. 1992;129:61–70. [PubMed] [Google Scholar]
- 46.Geraci JP, Mariano MS, Jackson KL. Amelioration of radiation nephropathy in rats by dexamethasone treatment after irradiation. Radiat Res. 1993;134:86–93. [PubMed] [Google Scholar]
- 47.Geraci JP, Sun MC, Mariano MS. Amelioration of radiation nephropathy in rats by postirradiation treatment with dexamethasone and/or captopril. Radiat Res. 1995;143:58–68. [PubMed] [Google Scholar]
- 48.Verheij M, Stewart FA, Oussoren Y, Weening JJ, Dewit L. Amelioration of radiation nephropathy by acetylsalicylic acid. Int J Radiat Biol. 1995;67:587–96. doi: 10.1080/09553009514550701. [DOI] [PubMed] [Google Scholar]
- 49.Aeolus Pharmaceuticals Aeolus Pharmaceuticals provides positive update on FDA meeting. [2014 Sep 18];Aeolus Pharmaceuticals [internet] 2012 Sep; ( http://mwne.ws/1wUIcW8)
- 50.Wang LW, Fu XL, Clough R, Sibley G, Fan M, Bentel GC, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res. 2000;153:405–10. doi: 10.1667/0033-7587(2000)153[0405:caceip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Bezjak A, Soyfer V, Yi Q, Sun A, Kane G, Waldron J, et al. Radiation pneumonitis in lung cancer patients - the neglected patient-related variables. Int J Radiat Oncol Biol Phys. 2005;63:S229. [Google Scholar]
- 52.Jenkins P, Watts J. An improved model for predicting radiation pneumonitis incorporating clinical and dosimetric variables. Int J Radiat Oncol Biol Phys. 2011;80:1023–9. doi: 10.1016/j.ijrobp.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins P, Welsh A. Computed tomography appearance of early radiation injury to the lung: Correlation with clinical and dosimetric factors. Int J Radiat Oncol Biol Phys. 2011;81:97–103. doi: 10.1016/j.ijrobp.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Molteni A, Wolfe LF, Ward WF, Ts'ao CH, Molteni LB, Veno P, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 55.Ward WF, Lin PJ, Wong PS, Behnia R, Jalali N. Radiation pneumonitis in rats and its modification by the angiotensin-converting enzyme inhibitor captopril evaluated by high-resolution computed tomography. Radiat Res. 1993;135:81–7. [PubMed] [Google Scholar]
- 56.Cohen EP, Fish BL, Moulder JE. Mitigation of radiation injuries via suppression of the renin-angiotensin system: Emphasis on radiation nephropathy. Curr Drug Targets. 2010;11:1423–9. doi: 10.2174/1389450111009011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: Importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–24. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 58.Kohl RR, Kolozsvary A, Brown SL, Zhu G, Kim JH. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–5. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]
- 59.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]