Abstract
Accelerated apoptosis of erythroid progenitors in β-thalassemia is a significant barrier to definitive therapy because the beneficial effects of fetal globin–inducing agents on globin chain balance may not be inducible in cells in which programmed cell death is established early. Accordingly, our objectives have been to identify methods to decrease cellular apoptosis and to identify orally tolerable fetal globin gene inducers. A pilot clinical trial was conducted to determine whether combined use of a fetal globin gene inducer (butyrate) and rhu-erythropoietin (EPO), the hematopoietic growth factor that prolongs erythroid cell survival and stimulates erythroid proliferation, would produce additive hematologic responses in any thalassemia subjects. Butyrate and EPO were administered in 10 patients. Novel fetal globin gene inducers that also stimulate erythroid proliferation were evaluated for pharmacokinetic profiles. Patients with β+-thalassemia had relatively low levels of endogenous EPO (<145 mU/mL) and had additive responses to administered EPO and butyrate. Patients with at least one β0 -globin mutation had higher baseline HbF levels (>60%) and EPO levels (>160 mU/mL), and three-fourths of these subjects responded to the fetal globin gene inducer alone. A few select fetal globin–inducing short-chain fatty acid derivatives that stimulated cell proliferation also had favorable pharmacokinetics. These studies identify a significant subset of thalassemia patients who appear to require exogenous EPO to respond optimally to any HbF inducer, as well as new therapeutic candidates that act on both cellular and molecular pathologies of β-thalassemia. Both approaches now offer excellent potential for tolerable, definitive treatment of β-thalassemia.
Keywords: thalassemia, short-chain fatty acid, erythropoietin, fetal hemoglobin, apoptosis, molecular signaling
Introduction
The β-thalassemia syndromes are characterized by deficiency of β-globin chains and excess α-globin, with consequent red cell membrane damage and rapid apoptosis of early erythroblasts developing in the bone marrow.1–8 It is well established that continued high expression of fetal globin results in less severe anemia and milder clinical courses.1–4 From analysis of globin chain ratios of thalassemia trait and thalassemia intermedia subjects, non-α (fetal and β) globin chains that balance α-globin by approximately 50% result in transfusion independence and milder clinical courses.1,2,5 Complications of iron overload develop decades later in patients with milder anemia who do not require regular transfusions than in those in whom regular transfusions are begun early in life.3 Induction of high-level expression of endogenous fetal (γ) globin therefore became a goal of the hemoglobin-switching field. Inducing endogenous fetal globin is appealing because the fetal globin genes are present and normally integrated in hematopoietic stem cells and function well.1,2,5
Rapid cellular apoptosis is well characterized in β-thalassemia from the precipitation of α-globin chains.6–8 Optimal hematologic correction of β-thalassemia may require improvement in the underlying erythroid cell survival or proliferation to allow an agent that induces fetal globin expression to act before the programmed cell death pathway is irrevocably established. Chemotherapeutic agents that stimulate fetal globin production, such as 5-azacytidine, decitabine, and hydroxyurea, all inhibit cell proliferation and cause cell growth arrest, which is known to promote irreversible apoptosis in cancer cells.9–17 The chemotherapy agents are also mutagenic, a drawback for conditions requiring long-term treatment.18,19 Several short-chain fatty acids (SCFAs) specifically induce transcription from the γ-globin gene promoter and in some patients can increase the efficiency of γ- globin mRNA translation.20–31 These effects do not require histone deacetylase (HDAC) activity, but HDAC inhibitors often are potent γ-globin inducers.32 However, those SCFADs that inhibit HDACs also inhibit cell proliferation, which is a limiting factor in thalassemia.17,32,33 Further, their rapid metabolism necessitates large oral doses or intravenous infusions that are difficult for many patients to tolerate long term. The SCFAs are typically not mutagenic.
Erythropoietin, the hematopoietic growth factor that stimulates red blood cell production, decreases apoptosis, and prolongs survival of erythroid cells, has increased total hemoglobin and hematocrit significantly in patients with γ-thalassemia in several trials, particularly in children, and without a significant increase in HbF in most, thereby increasing thalassemic erythropoiesis.1,4,34–37 EPO and SCFAs thus have potentially complementary activities for β-thalassemia. Accordingly, a pilot study was conducted to determine whether combined use of EPO and butyrate has additive effects in inducing hematologic responses in any subjects with β-thalassemia.38
A second area of investigation has focused on determining whether any other SCFA derivatives are (1) orally active in inducing fetal globin expression, (2) can increase erythroid cell proliferation or decrease the cellular apoptosis of thalassemic erythroid cells, and (3) have more favorable pharmacokinetics than that of butyrate or phenylbutyrate. Novel SCFADs, discovered through computational modeling, are being evaluated to identify potent fetal globin inducers that are resistant to metabolism and stimulate erythroid proliferation.33,39 These investigations have identified significantly more favorable candidates for definitive therapy of β-thalassemia.
Materials and Methods
Clinical Protocol and Subjects
A pilot trial of butyrate and erythropoietin was conducted to determine whether the two agents might have additive activity in a proportion of patients with β-thalassemia. Two courses of intensive arginine butyrate therapy were administered for 10 h/day, 5 days per week for 1 month, followed by 2 weeks without any therapy. After two cycles of this “induction” therapy, intermittent or “pulse” butyrate was administered on 4 consecutive days every other week, or a total of 8 days per month, for 3 months. An erythropoietin phase was then added to the butyrate regimen, with EPO given three times/week, at 300 U/kg of body weight, subcutaneously or intravenously, for 6 weeks and then at 500 U/kg three times weekly for 6 weeks. For patients with more severe anemia with hemoglobin levels less than 6 g/dL, the erythropoietin phase was administered prior to butyrate. As iron supplementation has been universally required for other patient populations to respond to EPO therapy, iron supplements were administered when ferritin levels declined by 50% in iron-overloaded subjects and were given to all subjects who were not iron overloaded.
To be eligible for the trial, patients were required to have a diagnosis of β-thalassemia intermedia with total hemoglobin less than 10 g/dL, and they must have been untransfused for at least 3 months. Eleven patients, 5–50 years of age, were initially enrolled. Four had been transfused regularly and were transfusion dependent; seven were transfused intermittently. The patients had a variety of molecular mutations with no predominant mutation being represented. Two patients could not be weaned off transfusions and were scored as nonresponders, and one patient was unevaluable due to early termination from the protocol. Complete blood counts, reticulocytes, fetal globin mRNA or chain synthesis, HbF and percentage of F cells, EPO levels, chemistry panels, and urinalyses were monitored once or twice per week (during administration of butyrate) using standard methods. Eight of the 10 patients responded to either butyrate alone or butyrate with erythropoietin, with a mean increase in total hemoglobin exceeding 2.5 g/dL, as shown in Table 1.
Table 1. Summary of hematologic responses to EPO + butyrate (AB) in the 8 of 10 patients who responded with an increase in total hemoglobin and hematocrit.
| Group | Baseline total Hgb (g/dL) | Total Hgb (g/dL) Mean peak response | Number responsive to EPO | |
|---|---|---|---|---|
|
| ||||
| AB | AB + EPO | |||
| β+-thalassemia Low EPO and HbF (EPO 28–143 mU/mL) | 7.3–9.4 | 1.1 | 2.7 | 3 of 4 |
| β0-thalassemia High EPO (>160 mU/mL), high HbF (>60%) | 5.3–6.5 | 2.8 | 2.6 | 1 of 4 |
Investigation of Other Candidate SCFADS
Novel SCFAD compounds that induce fetal globin expression, identified through medicinal chemistry and computational modeling, were studied in erythroid cells cultured from patients with β-thalassemia to determine their effects on cell proliferation and in growth factor–dependent hematopoietic cell lines, as previously described.33,39 Compounds that stimulated erythroid cell proliferation in culture were administered to baboons, and plasma levels of the test compounds were assayed by HPLC-MS as previously described.29 Studies of potential signaling pathways of proliferative compounds were undertaken as previously described.33
Results
The responses observed in the β-thalassemia subjects are summarized in Table 1. The patients in this small trial had two patterns of baseline HbF and EPO levels and two general patterns of response. Of the eight responsive patients, four had β+-thalassemia, with baseline HbF levels below 50%, and (relatively) low endogenous EPO levels (<145 mU/mL). Patients who had at least one β0-globin mutation had baseline HbF levels greater than 60% and baseline endogenous EPO levels above 160 mU/mL (range, 163–3577 mU/mL). In the high-EPO, high-HbF group, fetal globin mRNA or synthesis often increased within days of beginning therapy with butyrate. In patients with the lower baseline HbF levels, fetal globin mRNA increased more slowly—only after 3–4 weeks of treatment. HbF levels increased in seven patients, and non–α- to α-globin synthetic ratios also improved following butyrate treatment by 30–40% above baseline. Improved globin synthesis ratios were usually observed 2–4 days following a treatment course. Hematologic responses also followed two different patterns between the two groups. Patients with high baseline HbF and EPO levels had a mean increase in total hemoglobin of 2.8 g/dL (range 1.5– 3.9 g/dL), usually reaching a peak after intensive butyrate treatment, and did not respond further to additional EPO therapy (summarized in Table 1). Patients with β+ thalassemia mutations had minimal responses to butyrate with a mean increase in total hemoglobin (Hgb) of 1.1 g/dL above baseline, and a further 1.6 g/dL mean increase in total Hgb when rhu-EPO was added. Withdrawal of butyrate resulted in a decline in Hgb and hematocrit after 2–3 months in two of three patients in whom EPO was continued alone. However, one patient has been maintained on EPO alone for 4 years with a sustained hematologic response. Serum transferrin receptors, ferritin (in iron-overloaded patients), and EPO levels all declined during the 6-month pulse butyrate treatment phase and likely contributed to declines from the peak responses in total Hgb and hematocrit. Both therapeutics were well tolerated; there were no adverse events that warranted discontinuation of either therapeutic agent.
Butyrate therapy resulted in transfusion independence in three β-thalassemia subjects who have been treated for 2–7 years, even with intermittent administration of 8 days/month, and phenylbutyrate therapy has resulted in prolonged hematologic responses in thalassemia subjects with allo-antibodies.25 These courses illustrated that SCFADs could significantly improve thalassemic red cell survival, even with infrequent administration. Molecular modeling and virtual screening of chemical libraries produced 20 candidate SCFAD compounds that induced activity of the fetal globin gene promoter in reporter gene assays at 1- to 1.5-log lower concentrations than was required for butyrate.39 In erythroid cells cultured from subjects with β+-thalassemia, several novel compounds were found to (1) increase proportions of F cells by 20–40% above control conditions; (2) stimulate erythroid colony proliferation by 20–75% above colonies developing with growth factors alone; and (3) signal through prolonged phosphorylation of Stat5, the same pathway employed by EPO, but bypassing the EPO receptor and Jak-2.33
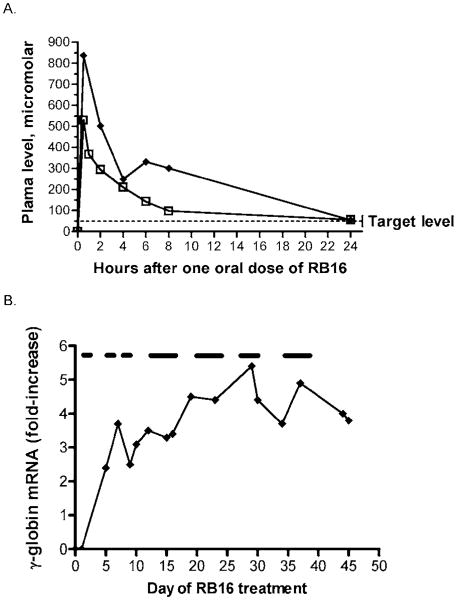
The candidate SCFAD compounds are undergoing evaluation for pharmacokinetics and pharmacodynamics in baboons, a primate model with metabolic systems close to those of humans. One promising new candidate compound, RB16, persists in the plasma for 24 h after single oral doses of 5–20 mg/kg in baboons, shown in Figure 1A. This is equivalent to a 1- to 2-mg/kg dose in an adult human and is substantially less than the 400- to 800-mg/kg doses required for butyrate and phenylbutyrate in humans. Fetal globin mRNA expression was induced four- to sixfold by this compound in a nonanemic baboon, as shown in Figure 1B.
Figure 1.
(A) Pharmacokinetic profiles of a novel SCFAD, RB16, administered at single oral doses of 10 mg/kg (open squares) and 20 mg/kg (closed symbols) in a baboon. The compound persisted in the plasma above the target concentration for more than 24 h following single oral doses. This target concentration induces fetal globin expression in erythroid cultures established from patients with β+-thalassemia, as shown by the dotted line. (B) Fetal globin mRNA before and during administration of RB16 in a nonanemic baboon. The test compound was administered once/day, 5 days/week during days 1–28 and for 4 days/week for another 2 weeks, as shown by the bars above the graph. A four- to sixfold induction of fetal globin mRNA above baseline was observed, as shown. The period of administration of the study compound is designated by the lines above the graph.
Discussion
The responses observed with EPO therapy are consistent with previous reports and provide simple criteria for readily selecting patients likely to respond to EPO therapy, those with relatively low HbF levels (<30%) and EPO levels (<145 mU/mL). The β+-thalassemia patients had low levels of endogenous EPO relative to their degree of anemia, and the patients responded optimally to the combination of a fetal globin-inducing agent and EPO. One patient with β0-thalassemia, a child who had an extremely high baseline EPO levels (>3000 mU/mL) also responded to EPO. In most of the responsive subjects, as ineffective erythropoiesis declined and total hemoglobin increased by 1–2 g/dL, EPO and ferritin levels also declined. These secondary physiologic responses may also respond to exogenous EPO therapy. The 75% response rates to EPO in the β+-thalassemia subjects strongly suggest that a trial of an EPO preparation is warranted, particularly in thalassemia intermedia, and before instituting a regular transfusion program with the predictable consequences of iron overload and transfusion-related infections. The only potential caveat may be the presence of extramedullary erythropoiesis in sites that could cause spinal cord compression, if further bone marrow expansion resulted. Such patients should be closely monitored if EPO is administered.
The major limitation of SCFAs as therapeutics has been rapid metabolism, which has necessitated large oral doses or intravenous infusions.21,2,27 Despite this short exposure, and despite intermittent administration, prolonged hematologic responses and even transfusion independence have resulted in some patients treated with arginine butyrate. These therapies currently are, however, difficult for long-term use. Pharmacokinetic studies of other SCFAD candidates in baboons now demonstrate that some derivatives would require doses of only 2–20 mg/kg, significantly less than the 400- to 800-mg/kg doses that have been necessary for arginine butyrate and sodium phenylbutyrate.
Most medical conditions require a combination of interventions for optimal management. The complexities of β-thalassemia suggest that definitive treatment to correct the red cell destruction will likely require more than one type of therapeutic regimen, particularly for patients with baseline hemoglobin levels below 6 g/dL. Optimizing cell survival with EPO or an EPO-mimetic is desirable. Intermittent use of agents that induce histone hyperacetylation (HDACi) and/or that hypomethylate DNA may or may not be necessary, in view of the recent report that hypomethylation in the Aγ-globin gene promoter in adult bone marrow cells is similar to that in fetal liver cells.40 However, such agents may produce higher-level responses by increasing chromatin accessibility in the Gγ-globin gene promoter and the locus control region. Although the concentrations necessary for histone deacetylase inhibition in vitro may be difficult to achieve in patients, hypomethylating agents and HDAC inhibitors (HDACi) have yielded synergistic responses in animal models and may well be important for patients with low baseline fetal globin expression.22 HDACi and hypomethylating agents should ideally be administered on an intermittent basis to avoid their strong inhibition of erythroid cell proliferation and the potent mutagenic effects of 5-azacytidine and decitabine.1,11,18,19,30,33
Finally, an oral, low-dose, proliferative SCFAD should be developed to maintain fetal globin gene expression and even to stimulate erythropoiesis. Several SCFADs have been found to accomplish both actions. The range of potential therapeutics with differing molecular actions for definitive treatment of β-thalassemia should now be capable of eliminating transfusion dependency in many patients.
Acknowledgments
This work was supported by grants HL-61208, DK-52962, DK-64535, and HL-78276 from the National Institutes of Health.
We greatly appreciate the donation of rhu-EPO (Procrit) provided by Ortho Biotech, Inc., the technical assistance of Marilyn Perry, and patient referrals received from Drs. Yih-Ming Yang, Antonio Piga, Griffin Rodgers, and Kenneth Bridges.
References
- 1.Steinberg MH, Rodgers GP. Pharmacologic modulation of fetal hemoglobin. Medicine. 2001;80:328–344. doi: 10.1097/00005792-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Gallo E, Massaro P, Miniero R, et al. The importance of the genetic picture and globin synthesis in determining the clinical and haematological features of thalassaemia intermedia. Br J Haematol. 1979;41:211–221. doi: 10.1111/j.1365-2141.1979.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearson HA, Cohen AR, Giardina PJ, et al. The changing profile of homozygous β-thalassemia: demography, ethnicity, and age distribution of current North American patients and changes in two decades. Pediatrics. 1996;97:352–356. [PubMed] [Google Scholar]
- 4.Rachmilewitz EA, Schrier SL. Pathophysiology of β-thalassemia. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin. Cambridge University Press; Cambridge: 2001. pp. 233–251. [Google Scholar]
- 5.Galanello R, Barella S, Turco MP, et al. Serum erythropoietin and erythropoiesis in high and low fetal hemoglobin beta-thalassemia intermedia patients. Blood. 1994;83:561–565. [PubMed] [Google Scholar]
- 6.Yuan J, Angelucci E, Lucarelli G, et al. Accelerated programmed cell death (apoptosis) in erythroid precursors of patients with severe beta-thalassemia. Blood. 1993;82:374–377. [PubMed] [Google Scholar]
- 7.Angelucci E, Lucarelli G, Yuan J, et al. Programmed cell death (PCD) and ineffective erythropoiesis in Cooley's anemia. Blood. 1996;88:22b. [Google Scholar]
- 8.Centis F, Tabellini L, Lucarelli G, et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precursors in patients with β-thalassemia major. Blood. 2000;96:3624–3629. [PubMed] [Google Scholar]
- 9.DeSimone J, Heller P, Hall L, et al. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;79:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar C, Travis W, Kan YW, et al. 5-Azacytidine treatment in a beta thalassemia patient unable to be transfused due to multiple allo-antibodies. Br J Haematol. 1989;74:467–468. doi: 10.1111/j.1365-2141.1989.tb07734.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaenisch R, Schnieke A, Harbers K. Treatment of mice with 5-azacytidine efficiently activates silent retroviral genomes in different tissues. Proc Natl Acad Sci USA. 1985;82:1451–1455. doi: 10.1073/pnas.82.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshy M, Dorn L, Bressler L, et al. 2-Deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96:2379–2384. [PubMed] [Google Scholar]
- 13.Ley TJ, DeSimone J, Anagou NP, et al. 5-Azacytidine selectively increases globin synthesis in a patient with beta+-thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 14.Lowrey CH, Nienhuis AW. Brief report: treatment with azacitidine of patients with end-state β-thalassemia. N Engl J Med. 1993;329:845–848. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- 15.Fucharoen S, Siritanaratkul N, Winichagoon P, et al. Hydroxyurea increases Hb F levels and improves the effectiveness of erythropoiesis in beta thalassemia/Hb E disease. Blood. 1996;87:887–892. [PubMed] [Google Scholar]
- 16.Hajjar FM, Pearson HA. Pharmacological treatment of thalassemia-intermedia with hydroxyurea. J Pediatr. 1994;125:490–492. doi: 10.1016/s0022-3476(05)83304-9. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri C, Stice L, Faller DV. Butyrate-induced G1 arrest results from p21-independent disruption of retinoblastoma protein-mediated signals. Cell Growth Differ. 1998;9:465–474. [PubMed] [Google Scholar]
- 18.Carr BI, Reilly JG, Smith SS, et al. The tumorigenicity of 5-azacytidine in the male Fischer rat. Carcinogenesis. 1984;5:1583–1590. doi: 10.1093/carcin/5.12.1583. [DOI] [PubMed] [Google Scholar]
- 19.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 20.Ginder G, Whitters MJ, Pohlman JK. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci USA. 1984;81:3954–3957. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrine SP, Ginder G, Faller DV, et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the -globin disorders. N Engl J Med. 1993;328:129–131. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- 22.Constantoulakis P, Knitter G, Stamatoyannopoulos G. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison of combination treatments with 5-AzaC and AraC. Blood. 1989;74:1963–1971. [PubMed] [Google Scholar]
- 23.Liakopoulou E, Blau CA, Li Q, et al. Stimulation of fetal hemoglobin production by short chain fatty acids. Blood. 1995;86:3227–3235. [PubMed] [Google Scholar]
- 24.Collins AF, Dover GJ, Luban NL. Increased fetal hemoglobin production in patients receiving valproic acid for epilepsy. Blood. 1994;84:1690–1691. [PubMed] [Google Scholar]
- 25.Collins AF, Pearson HA, Giardina P, et al. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:39–43. [PubMed] [Google Scholar]
- 26.Ikuta T, Kan YW, Swerdlow PS, et al. Alterations in protein-DNA interactions in the gamma-globin gene promoter in response to butyrate therapy. Blood. 1998;92:2924–2933. [PubMed] [Google Scholar]
- 27.Atweh GF, Sutton M, Nassif I, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93:1790–1797. [PMC free article] [PubMed] [Google Scholar]
- 28.Liakopoulou E, Li Q, Stamatoyannopoulos G. Induction of fetal hemoglobin by propionic and butyric acid derivatives: correlations between chemical structure and potency of Hb F induction. Blood Cells Mol Dis. 2002;29:48–56. doi: 10.1006/bcmd.2002.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pace BS, White GL, Dover GJ, et al. Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood. 2002;100:4640–4648. doi: 10.1182/blood-2002-02-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skarpidi E, Cao H, Heltweg B, et al. Hydroxamide derivatives of short-chain fatty acids are potent inducers of human fetal globin gene expression. Exp Hematol. 2003;31:197–203. doi: 10.1016/s0301-472x(02)01030-5. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg RS, Ji X, Sutton M, et al. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao H, Stamatoyannopoulos G, Jung M. Induction of human gamma globin gene expression by histone deacetylase inhibitors. Blood. 2004;103:701–709. doi: 10.1182/blood-2003-02-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boosalis MS, Bandyopadhyay R, Bresnick EH, et al. Short-chain fatty acid derivatives stimulate cell proliferation and induce STAT-5 activation. Blood. 2001;97:3259–3267. doi: 10.1182/blood.v97.10.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nisli G, Kavakli K, Vergin C, et al. Recombinant human erythropoietin trial in thalassemia intermedia. J Trop Pediatr. 1996;42:330–334. doi: 10.1093/tropej/42.6.330. [DOI] [PubMed] [Google Scholar]
- 35.Rachmilewitz EA, Aker M, Perry D, Dover G. Sustained increase in haemoglobin and red blood cells following long-term administration of recombinant human erythropoietin to patients with homozygous beta thalassemia. Br J Haematol. 1995;90:341–345. doi: 10.1111/j.1365-2141.1995.tb05156.x. [DOI] [PubMed] [Google Scholar]
- 36.Bourantas K, Economou G, Georgiou J. Administration of high doses of recombinant human erythropoietin to patients with beta-thalassemia intermedia: a preliminary trial. Eur J Haematol. 1997;58:22–25. doi: 10.1111/j.1600-0609.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 37.Singer ST, Sweeters N, Vichinsky E, et al. A dose-finding and safety study of darbepoetin alfa (erythropoiesis stimulating protein) for the treatment of anemia in patients with thalassemia intermedia. Blood. 2003;102:268a. [Google Scholar]
- 38.Perrine SP, Yang YM, Piga A, et al. Butyrate + EPO in beta thalassemia intermedia: interim findings of a phase II trial. Blood. 2002;100:47a. [Google Scholar]
- 39.Perrine SP, Boosalis MS, Emery DW, et al. A pharmacophore model for screening Hb F-inducing agents. Blood. 2003;102:122a. [Google Scholar]
- 40.Lowrey C. Epigenetic modifications of the human β-globin LCR core elements and γ-globin gene promoters. Blood Cells Mol Dis. 2005;34:104–105. [Google Scholar]