Abstract
The development of intravenous busulfan and its incorporation in the preparative regimens for allogeneic stem cell transplantation has changed transplantation for myeloid maligancies. Bypassing the oral route to achieve 100% bioavailability translated into improved control over drug administration, with increased safety and reliability of generating therapeutic busulfan levels, maximizing antileukemic efficacy. Busulfan-nucleoside analog-based conditioning chemotherapy, thus far represented by fludarabine, is becoming the conditioning chemotherapy regimen of choice for patients with acute myelogenous leukemia at many transplant centers. The use of busulfan-based conditioning is extending rapidly also to hematopoietic stem cell transplantation for lymphoid malignancies, genetic diseases and umbilical cord blood transplantation.
Keywords: Busulfan, conditioning regimens, hematologic malignancies, hematopoietic stem cell transplantation
Introduction
The bifunctional DNA alkylating agent Busulfan (Bu) is now widely used as an alternative to total body irradiation (TBI) in conditioning therapy for hematopoietic stem cell transplantation (HSCT). However, initially, oral Bu was used as palliative treatment of chronic myelogenous leukemia (CML) and other myeloproliferative disorders since the 1950s [1]. Several sulfonic-acid ester derivatives had been identified as having potent antitumor activity and being inhibitory of normal hematopoiesis. One such compound, 1,4-dimethanesulphonyloxybutane, (Myleran®, Bu), showed intense suppressive properties on myeloid cell proliferation and, “compared with X-Rays, it did not appreciably depress lymphocyte formation at a dose which causes a 50% or more decrease in the number of (circulating) neutrophils”[1]. The authors perceived a relative lack of adverse effects which allowed the more widespread use of Bu in patients with myeloproliferative disorders, mainly CML [1,2]. Later on, the use of Bu was gradually replaced from palliative treatment of myeloproliferative diseases to pretransplant conditioning. This emerged from the experience of Santos and Tutschka, who translated to humans the potent myeloablative effect of Bu observed in their murine aplastic anemia model [3]. These pioneer investigators combined oral Bu with cyclophosphamide (Cy), or BuCy, to use it as conditioning therapy for allogeneic HSCT in patients with AML [4]. Oral BuCy was quickly recognized as an effective pretransplant regimen for a wide variety of hematologic malignancies and non-malignant disorders, providing a desired alternative to the standard regimen of TBI and Cy (TBICy). A slightly revised regimen (oral BuCy2) remains a standard conditioning regimen for HSCT [5].
Lately however, myeloablative as well as reduced-intensity pretransplant conditioning with Bu in combination with the nuceloside analog fludarabine (BuFlu), is gradually replacing BuCy and TBICy as preferred conditioning regimens for patients with myeloid malignancies. Bu in combination with melphalan (BuMel) is another promising conditioning regimen for both myeloid and lymphoid malignancies. As we look forward to the future, nucleoside analogs of later generation like clofarabine, drugs with similar immunosuppressive capabilities as Flu but with greatly enhanced antileukemic properties, are likely to complement Flu in combination with intravenous (IV) Bu. Moreover, we envision an IV Bu-nucleoside analog platform to safely deliver other agents including chemotherapeutic drugs, immuno-modulatory and cellular therapies added pre- and/or post-transplant to further enhance the efficacy of preparative regimens and increase the benefit of HSCT.
I. Clinical Experience, Therapeutic Results and Toxicity
It has been more then 30 years since Thomas and colleagues published their results on 100 patients treated with TBI and high-dose Cy in a proof-of-principle paper, demonstrating that stem cell transplantation is not only feasible but may be curative for acute leukemia [6]. Moreover, this group established the use of TBICy as a conditioning regimen for such patients, using Cy at 50 mg/kg daily for 4 days in addition to 10 Gy TBI. Unfortunately, the price for this approach was a treatment-related mortality (TRM) in excess of 50%, signaling a need for improved safety of the procedure [6]. About the same time, Bleyer et al. reported a successful transplant after combination chemotherapy without TBI, demonstrating that TBI is not mandatory for a successful outcome of HSCT [7]. The use of TBI has subsequently become associated with a variety of immediate and long-term post-transplant complications, including retarded intellectual development and stunted growth in children, as well as secondary malignancies, cataracts and endocrine dysfunction, all of which support the development of alternative conditioning regimens for HSCT [8-10].
Santos et al. reported in 1983 on the replacement of TBI with high doses of Bu [4]. Busulfan, as the antileukemic agent, in combination with Cy as an immunosuppressive adjunct, proved to be an effective conditioning regimen [4]. This group treated 51 patients with AML with Bu at 1 mg/kg orally every 6 hours for 16 doses followed by Cy at 50 mg/kg IV daily for four days (BuCy) [4]. An every 6 hour schedule was arbitrarily chosen because Bu tablets were highly irritating to the gastric mucosa, thus emetogenic, and patients were required to take a large number of tablets to achieve the desired Bu systemic exposure (SE). These investigators showed a clear stratification of outcomes based on disease status at transplant: 44% of the patients in CR1 achieved stable long-term remission, as compared with 0% for patients with more advanced disease [4]. Overall, grade 2-4 acute graft-versus-host disease (aGVHD) developed in 44% of patients and chronic graft-versus-host disease (cGVHD) in 22%. Clinically significant hepatic failure, veno-occlusive disease (HVOD), occurred in 3 patients [4]. These results again demonstrated that TBI can be replaced in the pretransplant conditioning for acute leukemia patients. However, a transplant-related mortality (TRM) rate of 73% was reported in this study. Due to this high treatment-related toxicity (including, but not limited to HVOD), Tutschka and colleagues revised the preparative regimen for patients with leukemia [5]. This group reduced the Cy dose from 200 mg/kg to 120 mg/kg (60mg/kg daily administered over 2 days), while using the same dose of Bu (1 mg/kg every 6 hours for 16 doses) [5]. Fifty patients with leukemia (AML, ALL and CML) were treated with this modified regimen with remarkable results; neutrophil engraftment was achieved in 96% of patients after a median of 13 days. No one had severe mucositis, only one patient developed clinically significant, but reversible, HVOD, and the actuarial 3-year overall survival was 65% [5]. A surprisingly low incidence of infectious complications, coupled with a low incidence of GVHD was also noted [5]. This study showed that the Cy dose reduction did not compromise the antileukemic activity, but significantly reduced the toxicity of the conditioning regimen. These results compared favorably with TBICy, especially for patients with CML, established a new standard in conditioning chemotherapy for HSCT for patients with leukemia and prompted larger scale comparisons between the BuCy2 regimen and the more established TBICy regimen.
Investigators at the Fred Hutchinson Cancer Research Center (FHCRC) performed a study comparing oral BuCy2 with TBICy as conditioning for CML patients receiving allogeneic HSCT [11], and three other multi-institutional, randomized studies compared BuCy2 with TBICy as conditioning regimen in myeloid leukemias [12-14]. In an additional single-institution study, oral BuCy2 was compared with TBI-Etoposide [15]. A meta-analysis [16], and a long-term follow-up study of the first four studies [17] revealed similar results; Socie et al. reported the results of 488 patients, 316 with CML and 172 with AML, treated with ByCy2 or TBICy after a median follow-up of seven years. All patients received either Bu 1 mg/kg PO q 6 hours for 4 days, or 10 Gy TBI plus the same dose of Cy, 60 mg/kg/day for 2 days. There was no difference in projected 10-year overall survival between patients treated with BuCy2 and TBICy (65% versus 63%, respectively) in CML, whereas a numerical but statistically non-significant difference in survival was noted in AML patients, whose projected 10-year survival was 51% and 63% after BuCy2 and TBI-Cy, respectively. Further, there was no difference in extensive chronic GVHD between the two groups for either disease type. The frequency of HVOD was not reported. The incidence of cataracts was significantly higher in CML patients after TBICy, and this complication was also associated with cGVHD. The only other difference was a slightly higher incidence of (permanent) alopecia after Bu-based therapy. The overwhelming majority of patients in these studies were adults however, and the delayed growth and retarded intellectual development, which remain major concerns with TBI-based conditioning in children, could not be assessed. The CML study at the FHCRC showed similar outcomes as the European studies. None of these studies unfortunately had a long enough follow-up to address the development of secondary malignancies, that needs more than a decade of lead-time after radiation therapy, and which is being increasingly recognized after the use of involved-field (mantle) radiation therapy for Hodgkin’s lymphoma [18-20]. Moreover, a dramatic increase in the incidence of secondary solid tumors has also been observed after autologous transplant with TBICy for non-Hodgkin’s lymphomas [21], confirming data from the treatment of Hodgkin’s disease, which suggested that a combined chemotherapy-radiation program may have an increased incidence of malignancies than the chemotherapy-only approach (with alkylating agents) [7-9, 22,23].
While overall there was no major difference in outcomes after TBI- and Bu-based conditioning therapy in these large studies, BuCy2 slowly became accepted as a new standard conditioning regimen for myeloid leukemias and caused a shifting trend away from TBI in pretransplant therapy for most myeloid disorders, an expected result considering its ease of administration and the lack of need for a TBI facility when using chemotherapy alone.
The toxicity from virtually any ablative preparative regimen has been associated with development of HVOD [24-26]. Accordingly, HVOD and/or hepato-renal failure have been of concern with oral Bu administration in combination with Cy, and was commonly considered a “trade-mark” toxicity associated with high-dose Bu (both the original BuCy and BuCy2 regimen) [27-30]. Additionally, oral Bu is associated with a hepatic first-pass extraction effect that can result in locally high Bu concentrations in the portal-hepatic venous system, which conceivably may contribute to the development of HVOD [31]. However, in addition to Bu, Cy is clearly also hepatotoxic. The contributions of other alkylating agents to the emergence of HVOD, such as Cy or thiotepa, have been illustrated by several investigators [32-34]. Briefly, Hassan showed that the time interval between the last Bu dose and the first Cy dose in BuCy2 is of importance for the development of HVOD. If this interval was more than 24 hours, the risk of HVOD was significantly lower than if it were less than 12 hours [32]. McDonald and coworkers demonstrated a highly significant association between the blood levels of certain Cy metabolites and VOD after TBICy [33]. Finally, Przepiorka and coworkers demonstrated that replacing 25-30% of the Bu dose in BuCy2 with thiotepa (Thiotepa-BuCy) resulted in a HVOD incidence in excess of 30%, or at least as high as that reported in the literature with the standard BuCy2 in patients transplanted for high-risk hematological malignancies [34]. Together, these data illustrate that the development of HVOD is likely multifactorial. The findings of McDonald suggest that inter-individual differences in metabolic drug handling are of importance for development of HVOD, with Cy being a probable contributor to the overall risk. This risk should be expected to further increase with the use of two or more alkylating agents that utilize hepatic GSH stores for their detoxification. When such drugs are combined in close time proximity they jointly contribute to the depletion of hepatic GSH, resulting in an exacerbated risk for serious hepatic injury, HVOD, also known as sinusoidal obstruction syndrome (SOS) [33].
In addition to HVOD, neurotoxicity was associated with Bu in animals [35]. Convulsions in a patient receiving Bu were first reported by Marcus and Goldman [36]. The incidence of neurotoxicity after Bu-based conditioning therapy was subsequently estimated to be up to 10% in adults [37], and approximately 7 % in children [38]. Vassal reported that higher doses (> 600/m2 or 16mg/kg) were associated with an increased probability of neurotoxic manifestations [39]. Such adverse events have been correlated with the capacity of Bu to cross the blood-brain barrier and is manifested primarily as generalized seizures. A limited degree of plasma protein binding allows Bu, unlike other lipophilic alkylating agents like melphalan, to easily cross the blood-brain barrier and achieve levels in CSF that are similar to those in plasma [39,40]. An altered blood-brain barrier may further increase the susceptibility to Bu-associated neurotoxicity. Seizures are more common in older patients, and appear to be dose dependent both in adults and children [38,40,41]. In adults, seizures typically occur in the 3rd or 4th day of Bu administration, probably as a result of drug accumulation [36,40-44]. Even without overt seizure activity EEG abnormalities can occur in up to 60% of patients [42]. Various anticonvulsant medications have been used for seizure prophylaxis including phenobarbital sodium, benzodiazepines (clonazepam, lorazepam) and phenytoin [41,42,45-47]. Phenytoin has been widely used for seizure prophylaxis in patients receiving Bu as part of the conditioning regimen due to its non-sedating properties. An IV loading dose is commonly administered to achieve a therapeutic level (10-20 ng/mL), thereby avoiding the delay in achieving a therapeutic level, which is commonly seen with oral phenytoin. Hassan and colleagues showed that phenytoin used as prophylactic anticonvulsant caused a higher clearance of oral Bu as opposed to when diazepam was used and, therefore, suggested that drugs without microsomal enzyme-inducing properties should be used for Bu seizure prophylaxis [48]. Benzodiazepines have since been employed for prophylaxis by numerous investigators [46-48]. However, in spite of their use, occasional patients still develop seizures with benzodiazepine prophylaxis [49]. The sedation associated with benzodiazepines may be considered less desirable by some patients, and newer anticonvulsants like leviracetam, which does not interfere with the hepatic microsomal cytochrome P450 pathway, remain to be investigated for seizure prophylaxis in this setting.
These clinical data and related concerns about oral Bu toxicity formed the basis for our hypotheses that 1) an IV Bu formulation might cause less stress to the liver, since parenteral administration will alleviate the unpredictable oral drug bioavailability as well as circumvent the first-pass effect of oral drug, and 2) combining Bu with an alternative immunosuppressive agent without hepatic metabolism, i.e. replacing Cy with a nucleoside analog, should yield increased safety of the conditioning therapy, since only one of the two drugs is now dependent on hepatic GSH-stores for its detoxification.
The practical limitations in using oral Bu relate primarily to its unpredictable and erratic bioavailability due to variable intestinal absorption. This prompted the design of an IV Bu formulation to achieve absolute dose assurance associated with its controlled administration [50]. The pharmacokinetics of IV Bu was evaluated in a phase I clinical trial performed in 15 patients with hematologic malignancies [51]. The new preparation was evaluated in combination with oral Bu and Cy. An IV Bu dose of 0.8 mg/kg and a targeted AUC of 1100-1200 μmol/L per min were identified as equivalent to oral drug at an average of 1.0 mg/kg, and therefore considered for subsequent phase II study [51]. In this study, the average bioavailability of oral Bu was 69%, ranging from <10% to virtually 100%. These results were confirmed by the papers of Hassan et al. [52], and Schuler et al. [53], who estimated the average bioavailability of oral Bu to be in the order of 70-80%, using different parenteral reference formulations. A phase II clinical trial was performed in 61 patients with advanced hematologic malignancies (75% with active disease at transplant) treated with IV Bu (0.8 mg/kg every 6 hours × 16) followed by Cy (60 mg/kg IV daily × 2) [54]. The regimen was very well tolerated (day 100 TRM was less than 10%); however, 2 patients developed fatal HVOD, one of whom had a prior HSCT. Eighty-six percent of the patients achieved an AUC of 800-1500 μMol-min [54].
The combination of Cy with intravenous Bu administration appeared safer compared with that of oral Bu. So far, IV BuCy2 has been compared with oral BuCy2 in 5 retrospective studies, all showing superiority of IV BuCy2 with regards to the development of HVOD and early transplant-related mortality [55-59]. Kashyap observed a significantly lower incidence of HVOD in patients with hematologic malignancies treated with IV as compared with oral Bu (33% vs 8%) and a significantly lower day-100 TRM [55]. In multivariate analysis, the use of the oral formulation was the strongest predictor of the development of HVOD. Similar results were noted for patients with myeloid diseases (leukemias and MDS) [56-58] and lymphoid malignancies (non-Hodgkin’s lymphoma) [59]. Interestingly, Aggarwal et al reported, that in 49 patients who underwent autologous stem cell transplant for intermediate and high-risk non-Hodgkin’s lymphoma with pharmacokinetic monitoring (AUC 1000-1500 μMol-min), the use of IV Bu yielded not only a reduction in non-relapse mortality from 28% to 3%, but a significantly improved overall and progression-free survival were also noted with IV as compared with oral Bu [59]. The latter findings were supported by Dean and coworkers, who reported improved outcomes of NHL patients undergoing auto-HSCT using IV BuCy2 as compared with a reference group that had received the oral formulation [60].
The introduction of IV Bu with Cy appeared to improve the safety of the BuCy2 regimen, however early regimen-related toxicity was still of concern. It had become apparent through the work of McDonald and coworkers that Cy, used in high doses in the pretransplant setting, contributed to significant hepatotoxicity, regardless of whether Bu is incorporated in the regimen [25,33]. Thus, as the metabolites of Cy (especially o-carboxylethylphosphamide mustard) likely contribute to HVOD in the double alkylating BuCy conditioning regimen, the risk for this untoward effect could conceivably be decreased by substituting Cy with an immunosuppressive agent from a different class without hepatotoxicity [25,61]. Fludarabine (Flu) would fulfill these criteria; Flu is an antimetabolite nucleotide inhibitor of DNA synthesis with powerful immunosuppressive properties. It potentiates radiation- and alkylator-induced cytotoxicity through inhibition of DNA damage repair [62,63]. From these studies it can be deduced that Flu most likely has a similar synergistic interaction with Bu. Fludarabine was introduced in HSCT by Terenzi and colleagues, concerned about other adverse effects associated with high-dose Cy, such as hemorrhagic cystitis, interstitial pneumonia and cardiotoxicity, rather then hepatotoxicity, as they intended to use it in combination with TBI [64]. Fludarabine was equally immunosuppressive to Cy in mice, and due to significantly less adverse effects, it was an excellent candidate for replacement of Cy in preparative regimens for HSCT. Slavin and colleagues subsequently reported on a reduced-intensity conditioning regimen using oral Bu and Flu (BuFlu) as preparative regimen for 26 patients with hematologic malignancies and nonmalignant disorders undergoing matched sibling donor HSCT [65]. This reduced-intensity regimen was well tolerated; however relapse remained a major cause of treatment failure [66].
Bornhauser and colleagues used oral Bu in combination with Flu (Flu 30 mg/m2 IV once daily for 4 days followed by Bu 1mg/kg q 6 hours for another 4 days). The Bu dose was adjusted to target steady-state plasma levels of 900 ± 100 ng/mL (approximately 1,200-1,500 μMol-min). Fourty-two patients with MDS and CML were treated. Engraftment was achieved in all patients with very low day-100 regimen-related mortality. Twelve patients (29%) died of relapsed disease while the one-year TRM was 24% [67].
The long half-life of Flu allows once daily administration, and in combination with IV Bu has been shown to have low toxicity and an impressive antineoplastic activity [68,69]. The combination of Flu with Bu was developed to achieve effective myeloablation, optimize antileukemic activity and safety of the conditioning therapy [68,69].
Russell and colleagues initially reported on a myeloablative conditioning regimen using IV Bu-Flu and ATG in a convenient once-daily dosing [68]. Seventy patients with hematologic malignancies were treated with a combination of fludarabine 50 mg/m2 on days −6 to −2 and IV Bu 3.2 mg/kg IBW daily on days −5 to −2, and all patients received ATG, 4.5 mg/kg [68]. In a second, disease-specific study, performed at the M.D. Anderson Cancer Center (MDACC), Flu and IV Bu were given once daily with each dose of Bu following Flu to achieve synergistic cytotoxicity and high intracellular Bu concentrations [62,69]. Ninety-six patients with AML/MDS were treated in this study with a combination of fludarabine 40mg/m2 daily for 4 days followed immediately by IV Bu 130mg/ m2 daily for 4 days (−6 to −3). ATG was added only for matched unrelated donor- and one antigen mismatched sibling donor-patients [69]. More than 97% of the patients achieved primary engraftment in these two studies [68,69]. Complete chimerism was recorded in 70% by day 30, increased to 100% in 84% of patients by day 100, with the rest having a stable mixed chimera with a median of 98% donor cells. The 100-day TRM was less than 5%, and the incidence of serious aGVHD (grade III-IV) was less than 10% in both studies. Chronic GVHD was noted in 38% and 55% of the patients, respectively [68,69]. Overall, 34% of the AML patients relapsed in the MDACC study [69], while the relapse rates (21% and 26%) for patients with AML treated in CR1 were similar in both reports. DFS and OS were 74% and 75%, and 88% and 81% (at 2 years for patients in the first study and 1 year in the second study), respectively, for patients transplanted in any CR. Pharmacokinetic studies revealed that Bu was cleared in less than 24 hours without drug accumulation and with less than 20% interdose variability in clearance and AUC. The median calculated daily Bu AUC values were 4866 and 4980 μM-min respectively [68-70]. Stomatitis was the most common toxicity (grade 2 occurring in more than 50% of patients). The majority of patients in the first study, and 18% and 9% respectively in the second study, also had transient elevations of ALT and conjugated bilirubin within one to two weeks after transplant, without the development of HVOD. Only 3 of 166 (1.8%) patients treated in these two trials developed clinically significant HVOD, one of whom died. Neurotoxicity was also rare, 4% of patients developed a hand-foot syndrome in de Lima’s study and two patients developed seizures, one reported by Russell as having a subtherapeutic phenytoin level and one at 6 weeks after transplant likely related to tacrolimus [68]. Interstitial pneumonitis was not reported in either study.
These results suggested that IV BuFlu was effective and safe. Moreover, in a Bayesian comparison with IV BuCy, Andersson et al. demonstrated that IV BuFlu has very similar antileukemic activity to IV BuCy2 in AML/MDS [71]. Although no head-to-head comparison with BuCy2 exists, available retrospective data comparing BuFlu with BuCy2 strongly suggest that indeed IV BuFlu is safe and at least as effective as BuCy2 for patients with myeloid malignancies undergoing HSCT [71,72]. Recent matched-case data appear to challenge the latter when used for a multitude of hematologic malignancies, at least when ATG is routinely included as part of the conditioning regimen to alleviate GVHD, and when given in close proximity to the transplant to more effectively remove T-cells from the graft [73]. Thus, these authors confirmed the superior safety of the IV BuFlu-ATG regimen, yet claimed better antitumor activity of oral BuCy2 when used without ATG [73]. The addition of ATG may have resulted in increased in vivo T-cell depletion and could explain the different results between this study and the previous two manuscripts [71,72, 73]. A randomized comparison of IV BuFlu and BuCy2 could be necessary to resolve this conflict. Regardless, the impressive safety profile is getting the reduced-toxicity IV BuFlu regimens adopted as the new “standard of care” preparative regimen for myeloid diseases by many transplant centers from around the world.
II. Busulfan Pharmacokinetics, Systemic Exposure and Therapeutic Interval
The extreme variability in bioavailability and pharmacokinetics (PK) of oral Bu led to important discoveries of the relationship between PK and clinical endpoints such as engraftment and toxicity [27-30,38-40,74-76]. A large (retrospective) experience with the BuCy preparative regimen in adults established a Bu therapeutic window in the range of 900 to 1500 μMol-min, surrogate numbers representing the average AUC value after one of 16 doses in a typical every- 6-hour BuCy regimen, or a total course AUC of 14,400-24,000 μM-min [27-30,75,76].
Consequently, very low Bu AUC levels (below 900 μMol-min) have been correlated with graft failure and disease recurrence [29,30] whereas high Bu systemic exposure (Bu-SE) (above 1,500 μMol-min) have been associated with increased toxicity, especially hepatic and neurologic toxicity [27-32,36,38-40,44,52]. In particular, several studies concluded an association between HVOD and an elevated Bu-SE, represented by an AUC above 1500 μM-min [30]. However, not all investigators agree with this interpretation [77], and indeed, the development of HVOD appears to be multifactorial. HVOD occurs with increased frequency after previous hepatic irradiation [78], and/or the inclusion of other hepatotoxic chemotherapy (i.e. alkylating agents), like cyclophosphamide, whose hepatotoxic metabolites, acrolein and phosphorodiamidic mustard, have a clear etiologic role in the generation of this disease [33,79]. Furthermore, Johnson et al. reported that various isoenzymes of glutathione-S-transferase affect Bu metabolism to various degrees. Carriers of GSTA1*B had a 30% reduced Bu clearance and on average a 2.6-fold higher Bu AUC compared with non-carriers [80]. Finally, a transient (reversible) elevation of liver function tests predominantly, after methotrexate administration, has been noted in patients receiving IV Bu without other signs of HVOD [68,69] suggesting that a careful clinical evaluation and use of strict, standardized diagnostic criteria for HVOD (such as Jones criteria) are important for establishing a diagnosis of HVOD [24]. We previously reported that a significantly higher rate of aGVHD, gastrointestinal and hepatic toxicity, is associated with excessive Bu-SE. In contrast, post-transplant mortality showed a bimodal distribution, being significantly increased with both very high and low Bu-SE, thereby confirming the presence of a therapeutic interval for IV Bu of approximately 950-1520 μMol-min in the prototype IV BuCy2 regimen [81]. This therapeutic interval was the only factor predictive of survival in CML patients transplanted using the IV BuCy2 therapy. The availability of detailed pharmacokinetic information in clinical Bu dosing has been reported to improve patient outcome when applied with both oral and IV Bu, even though the erratic bioavailability of oral Bu has put the value of therapeutic dose monitoring into question [77]. A carefully controlled study at the FHCRC by Deeg and colleagues reported clinical outcomes in 50 patients between the ages or 55 and 66 transplanted for MDS [82]. The conditioning regimens included TBICy, Cy-fractionated TBI, (oral) BuTBI and targeted (oral) BuCy2. Sixteen patients received oral Bu targeted to average steady-state plasma levels of 600-900 ng/mL (approximately 900-1,350 μMol-min) and Cy. The cohort receiving PK-targeted oral Bu with Cy had the lowest non-relapse mortality and best survival [82]. The discrepancy in opinion about the predictive value of oral Bu-derived PK parameters likely resides in the up to 3-fold variability in dose-to-dose AUC in any patient receiving oral drug [83]. Therefore, to somewhat accurately estimate the total course AUC after oral dosing, a minimum of 4-5 complete plasma concentration profiles may be necessary after a typical 16-dose regimen [75, 84]. This necessitates not only in-house access to PK-laboratory services with the same day turn-around PK reporting, but also requires very dedicated staff to procure and analyze all samples (an estimated 5-10 samples per dosing profile) that have to be included in the analytic profile from individual patients.
While high variability of Bu-SE when combined with Cy has been associated with adverse outcomes in adults, such a relationship between Bu exposure and HVOD has not been clearly established in children [85]. Still, some investigators insist that targeted Bu-SE, AUC, does improve outcomes in pediatric patients, and have demonstrated a higher engraftment rate and lower incidence of HVOD with a consistently utilized therapeutic drug monitoring (TDM) strategy [85-87]. Again, the application of TDM in the setting of oral drug administration is fraught with the extremely labor-intensive need for serial complete PK dosing profiles after consecutive Bu doses to provide reliable and reproducible data [75,84]. This area potentially represents the most pronounced advantage for a parenteral Bu formulation with its minimal inter-dose variability in PK parameters [70].
The retrospective confirmation of an association between Bu-PK and clinical outcome after HSCT formed an incentive for therapeutic drug monitoring (TDM), which was introduced to reduce the highly variable Bu-SE, thereby improving the safety of (oral) Bu-based high-dose chemotherapy. However, when accuracy of dosing and rapid onset of action is important, PK accuracy is always superior for IV versus orally administered drugs [88]. We postulated that a parenteral Bu formulation would in itself provide an improved tool for safe myeloablative conditioning therapy since it circumvents both unpredictable intestinal absorption and the hepatic first-pass extraction [31]. It also allows TDM to be implemented to further reduce inter-patient variability due to differences in metabolic drug clearance. Subsequently, precise Bu delivery was confirmed to be even more important than previously thought, not only in relation to early regimen-related toxicity and the development of aGVHD but also in predicting the likelihood of being alive beyond 1 year after HSCT [81]. More recently, the IV Bu-Flu combinations are promising to be safer overall than Bu with Cy or other alkylating agents. It is conceivable, that a therapeutic interval will exist for IV Bu in combination with nucleoside analogs (Flu) as well. Indeed, Geddes et al indicated that a Bu-SE / AUC in excess of 6000 μMol-min daily, or a total course AUC in excess of 24,000 μMol-min, was associated with inferior survival [89], but additional work will be required to establish the exact boundaries of such an IV Bu therapeutic interval, when delivered in combination with a nucleoside analogs.
III. Development of IV Busulfan and Comparison with the Oral Drug
In (myeloablative) pretransplant chemotherapy dosing accuracy is of major importance, and it is therefore always preferable to use parenteral drug formulations [87]. The introduction of IV Bu allowed both optimized safety and detailed PK assessments without resorting to the extremely labor-intensive schedules that are required for realistic PK-modeling with oral Bu [75,84]. We and others proposed that parenteral Bu would improve the safety of the conditioning regimen, as the 100% dose assurance of IV Bu guarantees that therapeutic drug monitoring can be optimized. As is the case with all PK assaying, however, reliable PK information can only be acquired if attention is also paid to a multitude of details including the infusion (priming the tubing with drug, not saline, all the way to the patient, infusion by controlled-rate pump, and finally disconnecting all of the tubing and pump cassette at the end of infusion without the use of “saline chasers” to “clean the line from drug”). Meticulous recording of infusion and sampling times also has to be exercised. Recently published studies have confirmed that the variability in PK parameters after IV Bu dosing is significantly lower than that recorded with the oral formulation [54,68-70]. In contrast, the conclusion may be that use of the respective IV and oral Bu formulations yield the same (major) variability in PK parameters as those reported in the oral Bu literature [90].
At least three different Bu solvent systems have been utilized clinically [50,53,91,92] by independent investigators to estimate oral drug bioavailability by comparing the systemic exposure represented by AUC, after a predetermined oral dose with that of an IV reference dose with 100% bioavailability, in the same patients. All arrived to a similar conclusion, namely that, in adults, the average bioavailability of oral Bu is 70 to 80% and collectively, these reports unequivocally established that the IV Bu dose equivalent of 1mg/kg oral Bu would be 0.7-0.8 mg/kg [51-53]. The 0.8 mg/kg dose has subsequently been used in several phase II studies of IV Bu [54, 56-60, 71].
IV. Busulfan-based Conditioning Therapy in Pediatric Transplantation
Concerns about delayed growth and retarded intellectual development have been associated with the use of TBI in children, and it influenced a gradual shift to chemotherapy-only conditioning in pediatric transplantation. Busulfan has frequently been included in conditioning regimens used in pediatric HSCT since early 1980s when the BuCy regimen was introduced. Significant variability in Bu clearance was demonstrated in children [38,39,75,84,86,93]. The apparent plasma clearance rates in younger children can be up to 4-5 times higher than in adults, significantly lower Bu-SE with oral Bu administration when the dose range used was the same as originally used in adults. Thus, the dosing regimen of 1 mg/kg every 6 hours for 4 days was associated with very few toxic adverse effects, however predisposed pediatric patients to an increased risk for relapse and primary graft failure [75,84,85]. Various mechanisms have been proposed for this difference in clearance, i.e. altered intestinal drug absorption, increased hepatic first pass clearance rate in younger children, and an increased volume of distribution. These mechanisms could contribute to the lower AUC values consistently noted in children [94-99]. Further, Gibbs and colleagues demonstrated that children younger than 4 years appear to have a 2-4 times enhanced ability to metabolize Bu in comparison with adults [100]. This higher clearance is probably (at least in part) due to an increased formation of Bu-GSH conjugate in both liver and the upper intestinal wall mucosa, which may contribute to a greatly enhanced clearance of oral Bu, as an important mechanism by which children metabolize Bu more efficiently than adults [99,100].
Several studies suggested that dosing based on BSA may be more appropriate to obtain an AUC closer to adult values [84,96-99]. Vassal and colleagues proposed a dose of 600 mg/m2, to arrive at a similar Bu-SE to the AUC values seen in adults, and similar adverse effects profile as recorded with the more commonly used course dose of 16mg/kg dose [99]. However, even with this approach, a wide inter-patient variability was noted, ranging from 3,500 to13,000 ng.h/mL (approximately 850-3,300 μMol-min) [99].
The original dosing schedule of Bu administered at 1 mg/kg PO every 6 hours over 4 days was gradually modified in children to improve the safety and efficacy of drug delivery in response to the particularities related accelerated Bu clearance seen in pediatric patients. Shaw and coworkers suggested that a single daily oral Bu dose (either 4 mg/kg or 600mg/m2) would be equivalent to divided doses and showed similar pharmacokinetic data except to a predicted four-fold higher peak concentration [101]. They suggested that this would be more convenient without increasing the risk of serious adverse effects. The once-daily dose delivery schedule yielded Bu plasma peak concentrations reached approximately two hours after administration. The drug was virtually completely eliminated at 24 hours in the majority of patients. Moreover, when the AUC was used to calculate the Bu-SE, no significant differences were found between younger and older children. This contrasted with the 1mg/kg divided dosing schedule, which was associated with a significantly lower Bu-SE in younger children [101,102].
Oral Bu is associated with a wider inter- and intra-patient variability in children than in adults [52,97,100-102]. Therefore, controlling Bu-SE in children became even more important in order to avoid the highly variable exposures that predispose to serious adverse events, i.e. graft rejection and leukemic recurrence [75,84,93]. Targeting Bu to AUC values between 900 and 1500 μMol-min optimized the chance for engraftment and reduced the risk for severe toxicity, similar to that seen in adult population [93]. Accepting that there is a continuous variation in Bu clearance inversely correlated with weight in children, Vassal recently proposed a refined dosing schedule based on body weight for centers that do not have access to PK monitoring. This group administered IV Bu in combination with either melphalan or Cy in 55 patients on an every 6 hour schedule for 4 days, at 5 decrementing doses for a progressively increasing body weight, on the presumption that a continuous decrease of Bu clearance requires a gradual decrease in the dose administered. Doses ranging from 1.2 mg/kg to 0.8 mg/kg were administered to children weighting from less than 9 kg to more than 34 kg. The great majority of patients (91%) achieved an AUC in the targeted range with low inter-patient variability [93]. In this context, it may also be of interest to note the retrospective comparison between TBICy and oral BuCy in a matched pair analysis of pediatric patients having allogeneic SCT for ALL from the IBMTR [104]. This report strongly favored TBICy over oral BuCy, mostly without PK-guidance, in reference to OS, DFS and TRM [104]. It is important to note that, first, the introduction of IV Bu and then the replacement of Cy with Flu in the IV BuFlu and IV BuFlu-ATG regimens have contributed to drastically reduced TRM and improved OS and DFS for adults with AML/MDS and ALL [68,69,106]. Second, in pediatric SCT the IV Bu has made it easier to accurately utilize the TDM concept to achieve dosing accuracy with consistent systemic exposure. More recent reports also show an improved OS and DFS for patients receiving IV Bu-based conditioning therapy when compared with historical oral Bu controls. Additionally, Worth and colleagues reported excellent 10-year overall and disease-free survival in high-risk pediatric patients with AML/MDS and ALL. In particular, their engraftment rate of more than 90% and a 10-year DFS of 60% in a subpopulation of patients who received a single cord blood transplant for advanced leukemia are encouraging [105]. Finally, the data of Russell et al., utilizing their modified Bu- BuFlu-ATG-TBI regimen in adults with ALL (see below) [106], together with the emerging concerns about long-term sequelae of TBI in pediatric patients suggest that it may be time to re-evaluate the purported superiority of TBICy with that of an IV Bu-based conditioning regimen when optimized within the realm of TDM for pediatric patients.
V. Current State-of-the-art and Future Developments with IV Busulfan-based Conditioning Therapy
Available clinical results indicate, that the IV BuFlu conditioning regimens are safe and effective, and are being adopted as “standard of care” preparative regimens for myeloid diseases by many transplant centers. Although no head-to-head comparison with BuCy2 exists, available retrospective data comparing BuFlu with BuCy2 suggest that BuFlu is indeed safer and at least as cytoreductive as BuCy2 for patients with hematologic malignancies undergoing hematopoietic stem cell transplantation. Three retrospective studies performed in the past few years compared IV BuFlu with BuCy2 [71-73]. In one of them, Bredeson and colleagues reported a retrospective matched-pair analysis of 120 patients treated IV BuFlu plus ATG with 215 control patients treated with oral BuCy2 [73]. This analysis partly confirmed the dramatic differences in treatment-related mortality (12% versus 34%, P<0.001) and grades II-IV aGVHD (15% versus 34%) in patients treated with IV BuFlu-ATG as compared with those receiving BuCy2. However, they found a higher relapse rate for patients receiving IV BuFlu-ATG, translating in a similar overall survival at 5 years (58% IV BuFlu-ATG and 51% BuCy2), although the 100-day-, 1 year-, and 3 year-survival rates were greater and reached near statistical significance in patients treated with IV BuFlu-ATG [73]. The effaced difference in outcomes between IV BuFlu-ATG and BuCy2 regimens at 5 years is not entirely clear, presumably a cumulative relapse rate for indolent diseases like multiple myeloma, CML and some lymphomas may contribute, as diseases like AML or MDS are less likely to recur at 3 or more years after HSCT. It may be of importance to remember that the patients in this study had a variety of hematologic malignancies. Therefore, comparisons of different conditioning regimens may benefit from being disease-specific, in order to address whether anti-tumor efficacy is the same for regimens used for patients in different diagnostic subcategories. Furthermore, the described IV BuFlu- ATG program uses ATG including a dose delivered on the day of the graft infusion, such that this cohort may mimic the outcomes of a T-cell depleted transplant, where a higher relapse rate is commonly observed. There was no indication that ATG was used in the oral BuCy2 group.
A variant of the BuFlu-ATG regimen was explored recently by Russell and coworkers [106]. They treated 64 adult patients with acute leukemia in first or second remission with IV BuFlu and 400 cGy TBI. This variant of their previously reported regimen added TBI at 200 cGy given twice on days −1 or day 0, to enhance the antileukemic effect of the IV BuFlu-ATG regimen. All patients engrafted with a transplant-related mortality of only 3%, similar to the data previously reported [68]. An impressive projected DFS at 3 years of 83% ± 6% for AML and 65% ± 10% for ALL was reported [106]. Although the numbers were limited, the results are encouraging, especially for ALL patients, as a recent large retrospective analysis had shown only a modest 25% overall survival after the TBICy or (oral) BuCy2 conditioning regimens [107].
BuFlu conditioning has also been tested in the context of unrelated umbilical cord blood transplantation (UCBT), both with the drugs given sequentially and in the standard fixed-dose IV BuFlu (Bu 130 mg/m2/day and fludarabine 40 mg/m2/day × 4 days) regimen, as well as with PK-guided IV Bu delivery [108]. These IV BuFlu variant regimens performed poorly in patients receiving an UCBT; from 8 (out of 10 total) patients who were evaluable for engraftment, and who received a double cord transplant for myeloid malignancies in CR, only 2 engrafted with donor derived hematopoiesis. No ATG was administered in this study, which could have contributed to an excessive graft failure rate. These results are in agreement with (unpublished) data from MDACC, where only 6 of 11 UCB recipients conditioned with PK-guided IV BuFlu engrafted. However, the addition of a low dose of thiotepa (TT), to the IV BuFlu regimen as reported by Sanz and colleagues [109], demonstrates that a modified IV BuFlu regimen could successfully be used for UCBT. This group treated 73 patients with hematologic malignancies, (65 with acute leukemia and MDS) with thiotepa-BuFlu, and used ATG with cyclosporine and steroids as GVHD prophylaxis. The (presumed) improved eradication of host T-cells by the addition of thiotepa dramatically improved engraftment. Only single cords were used in this study and about 90% of the patients successfully achieved engraftment [109]. The rate of grade II-IV aGVHD was only 16% and day +100 TRM was 14% [109]. Another approach was used by Russell and coworkers who utilized their IV BuFlu-ATG regimen supplemented with 200 cGy TBI for 2 doses, with the radiation therapy given the day before umbilical cord blood graft infusion [106]. Using also single unrelated cord blood grafts these investigators achieved engraftment in 12/12 evaluable patients before transplant day 30. (Russell, J., personal communication, November, 2008).
Busulfan-Melphalan (BuMel) Conditioning Chemotherapy
Another approach to avoid the toxicity of added Cy to Bu conditioning while maintaining a high degree of myeloablation was to replace Cy with melphalan (Mel), in a combination to treat (primarily) lymphoid malignancies, especially ALL for which current conditioning regimens provide disappointing results [108]. The BuMel has been evaluated both in the autologous and allogeneic stem cell transplant setting, in adults and children [110-115]. These various regimens used in single arm phase II studies have provided promising results, and controlled studies are warranted. For instance, in an ongoing trial, Kebriaei and coworkers are evaluating once daily IV Bu in combination with melphalan (70 mg/m2 daily for 2 doses) for ALL and advanced high-grade lymphomas [115]. While this combination was well tolerated, disease-control in the ALL-subpopulation was still too early to fully evaluate, however in a cohort of 30 ALL patients the 1-year survival was approximately 80% (Kebriaei, P. personal communication, November, 2008). This combination regimen appears at least as good as what would be expected with TBICy in a patient population with and average age of about 35 years [106,107,115]. Furthermore, Wall and coworkers reported recently on the use of BuMel-ATG regimen as conditioning for unrelated umbilical cord blood transplantation in pediatric patients [116]. Again, the regimen was well tolerated, engraftment of granulocytes was achieved in 60% of patients by day BMT +42, and a one-year survival rate was 47%.
Busulfan - Alternative Nucleoside Analogs in Pretransplant Conditioning
Clofarabine and nelarabine are newer purine analogues, which, similar to Flu are active in various hematologic malignancies. Both drugs have been recently granted FDA approval for treatment of advanced ALL/lymphoma [117,118]. The immunouppressive and engraftment promoting capability of these nucleoside analogs remains unconfirmed, although the molecular structure of clofarabine suggests that it is likely to be at least comparable with that of Flu. To improve the cytoreductive effect of BuFlu regimen, IV Bu is being combined with clofarabine, in ongoing early trials [119]. Twenty-five patients with advanced AML/MDS were treated at MDACC with a combination of IV Bu-clofarabine +/− Flu. All evaluable patients engrafted uneventfully, and when assessed approximately at one month and 3 months post transplant the T-cell chimerism appeared to be at least as high as that achieved with the BuFlu combination. Although it is too early to evaluate disease-control, the preliminary data demonstrate the potential of Clo to be used as an alternative to Flu in combination with Bu in pretransplant conditioning therapy.
Busulfan-nucleoside Analog as a Platform for Future Conditioning Regimens
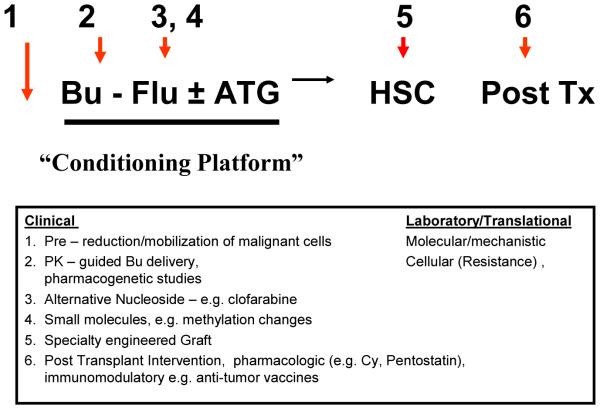
Intravenous Bu (± pharmacokinetic monitoring) allows precise drug delivery and control of systemic exposure, to minimize adverse effects and optimize the antitumor effect, and, in combination with Flu, represents a safer conditioning regimen for transplantation. The consideration of IV BuFlu as an emerging platform technology can be anticipated to lead into investigation of novel strategies to further improve outcomes of transplantation, as exemplified in Figure 1. First, improvement of the cytoreductive effect could be potentially accomplished by the addition of mobilizing agents, such as plerixafor, based on hypothesis that leukemic cells are more sensitive to chemotherapy when dissociated from the marrow stroma; Second, demethylating agents are of importance for the development of drug resistance in myeloid leukemia (MDS, AML). By adding such agents like azacitidine or decitabine to manipulate the methylation status of malignant cell DNA, an increased sensitivity of the malignant cells could be achieved; Third, the addition of other chemotherapeutic agents such as thiotepa or low-dose-TBI might enhance anti-leukemic activity for lymphoid malignancies, and as well as promote engraftment after umbilical cord blood transplantation; Fourth, the reduced toxicity of the conditioning regimen now safely allows the use of post-transplant immunomodulation (i.e. cyclophosphamide or pentostatin) to reduce the incidence of GVHD and allow the use cellular therapy after transplant.
Figure 1.
Potential peri-transplant interventions added to the IV BuFlu conditioning regimen for hematopoietic stem cell transplantation.
In conclusion, much has accomplished over the past 30 years in conditioning regimens for hematopoietic stem cell transplantation. Intravenous busulfan in combination with fludarabine for patients with myeloid malignancies in first complete remission is associated with more than 80% long-term survival due to improvement in safety of chemotherapy administration and decreased treatment-related mortality [68,69,106]. Available data proves that IV BuFlu (±ATG) is a safer and at least as effective preparative regimen as compared with either of the BuCy2 variants or TBICy, although there are no head-on comparisons available. The BuFlu regimen represents an important step forward in conditioning for HSCT, especially for myeloid malignancies, and can conceptually be considered as a platform for developing improved preparative regimens for lymphoid malignancies, alternative donor transplantation, as well as for other subgroups of high-risk patients for which outcomes are largely unsatisfactory using the available conditioning regimens.
Acknowledgments
This work was supported by Supported by National Institute of Health grants 2PO1 CA55164 and 2P30CA16672-26, and the Stephen L. and Lavinia P. Boyd Fund for Leukemia Research.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors are greatly indebted to the nursing staff of the inpatient and outpatient transplant care centers, research nurses and members of the stem cell transplant coordinator staff.
B. S. Andersson is a consultant to Otsuka America, Inc.
References
- 1.Haddow A, Timmis GM. Myeleran in chronic myeloid leukemia. Chemical constitution and biological action. Lancet. 1953;31:207–208. doi: 10.1016/s0140-6736(53)90884-8. [DOI] [PubMed] [Google Scholar]
- 2.Galton GA. Myeleran in chronic myeloid leukemia. Results of treatment. Lancet. 1953;31:208–213. doi: 10.1016/s0140-6736(53)90885-x. [DOI] [PubMed] [Google Scholar]
- 3.Santos GW, Tutschka PJ. Marrow transplantation in the busulfan-treated rat: preclinical model of aplastic anemia. J Natl Cancer Inst. 1974;53:1781–1785. [PubMed] [Google Scholar]
- 4.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 5.Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for acute leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70:1382–1388. [PubMed] [Google Scholar]
- 6.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–533. [PubMed] [Google Scholar]
- 7.Bleyer AW, Blaese RM, Bujak JS, Herzig GP, Graw RG. Long-term remission from acute myelogenous leukemia after bone marrow transplantation and recovery from acute graft-versus-host reaction and prolonged immunoincompetence. Blood. 1975;45:171–181. [PubMed] [Google Scholar]
- 8.Curtis RE, Rowlings PA, Deeg JH, et al. Solid cancers after bone marrow transplantation. N Eng J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 9.Socie G, Curtis RE, Deeg JH, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 10.Baker SK, DeFor TE, Burns LJ, Ramsey NKC, Neglia JP, Robinson LL. New malignancies after blood and marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 11.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulphan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- 12.Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- 13.Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplntation Group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- 14.Devergie A, Blaise d, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM) Blood. 1995;85:2263–2268. [PubMed] [Google Scholar]
- 15.Blume KG, Kopecky KJ, Henslee-Downey JP, et al. Blood. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
- 16.Hartman AR, Williams S, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998;22:439–443. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 17.Socie G, Clift RA, Blaise D, et al. Busulfan plus cyclophosphamide compared with total- body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–3574. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 18.Van Leeuwen FE, Klokman WJ, Hagenbeek A, et al. Second cancer risk following Hodgkin’s disease: a 20-year follow-up study. J Clin Oncol. 1994;12:312–325. doi: 10.1200/JCO.1994.12.2.312. [DOI] [PubMed] [Google Scholar]
- 19.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood or adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S, Yasui Y, Robinson LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 21.Brown JR, Yeckes H, Friedberg JW, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2208–2214. doi: 10.1200/JCO.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 22.Dores GM, Metayer C, Curtis R, et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Travis LB, Hill DA, Dores GM, et al. Breast cancer risk following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 24.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 25.McDonald GB, Hinds MS, Fisher LB, et al. Venoocclusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. [PubMed] [Google Scholar]
- 27.Grochow LB, Jones RJ, Brundett RB, et al. Pharmakokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25:55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 28.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20(Supl4):18–25. [PubMed] [Google Scholar]
- 29.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 30.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 31.Peters WP, Henner WD, Grochow LB, et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res. 1987;47:6402–6406. [PubMed] [Google Scholar]
- 32.Hassan M, Ljungman P, Ringden O, et al. The effect of busulfan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 33.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 34.Przepiorka D, Khouri I, Thall P, et al. Thiotepa, busulfan and cyclophosphamide as a preparative regimen for allogeneic transplantation for advanced chronic myelogenous leukemia. Bone Marrow Transplant. 1999;23:977–981. doi: 10.1038/sj.bmt.1701764. [DOI] [PubMed] [Google Scholar]
- 35.Deeg HJ, Schuler US, Schulman H, et al. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999;5:316–321. doi: 10.1016/s1083-8791(99)70007-8. [DOI] [PubMed] [Google Scholar]
- 36.Marcus RE, Goldman JM. Convulsions due to high-dose busulphan. Lancet. 1984;2:1463. doi: 10.1016/s0140-6736(84)91649-0. [DOI] [PubMed] [Google Scholar]
- 37.Santos GW. Busulfan (Bu) and cyclophosphamide (Cy) for marrow transplantation. Bone Marrow Transplant. 1989;4(Suppl 1):236–239. [PubMed] [Google Scholar]
- 38.Vassal G, Deroussent A, Hartmann O, et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–6207. [PubMed] [Google Scholar]
- 39.Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol. 1989;24:386–390. doi: 10.1007/BF00257448. [DOI] [PubMed] [Google Scholar]
- 40.Hassan M, Oberg G, Ehrsson H, et al. Pharmacokinetic and metabolic studies of high- dose busulphan in adults. Eur J Clin Pharmacol. 1989;36:525–530. doi: 10.1007/BF00558081. [DOI] [PubMed] [Google Scholar]
- 41.Meloni G, Raucci U, Pinto RM, Spalice A, Vignetti M, Iannetti P. Pretransplant conditioning with busulfan and cyclophosphamide in acute leukemia patients: neurological and electroencephalographic prospective study. Ann Oncol. 1992;3:145–148. doi: 10.1093/oxfordjournals.annonc.a058131. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi R, Watanabe N, Iguchi A, et al. Electroencephalogram abnormality and high- dose busulfan in conditioning regimens for stem cell transplantation. Bone Marrow Transplant. 1998;21:217–220. doi: 10.1038/sj.bmt.1701076. [DOI] [PubMed] [Google Scholar]
- 43.Martell RW, Sher C, Jacobs P, Monteagudo F. High-dose busulfan and myoclonic epilepsy. Ann Intern Med. 1987;106:173. doi: 10.7326/0003-4819-106-1-173_1. [DOI] [PubMed] [Google Scholar]
- 44.Sureda A, Perez de Oteyza J, Garcia Larana J, Odriozola J. High-dose busulfan and seizures. Ann Intern Med. 1989;111:543. doi: 10.7326/0003-4819-111-6-543_2. [DOI] [PubMed] [Google Scholar]
- 45.Grigg AP, Shepherd JD, Phillios GL. Busulphan and phenyoin. Ann Intern Med. 1989;111:1049–1050. doi: 10.7326/0003-4819-111-12-1049_2. [DOI] [PubMed] [Google Scholar]
- 46.Meloni G, Nasta L, Pinto RM, Spalice A, Raucci U, Iannetti P. Clonazepan prophylaxis and busulfan-related myoclonic epilepsy in autografted acute leukemia patients. Haematologica. 1995;80:532–534. [PubMed] [Google Scholar]
- 47.Chan KW, Mullen CA, Worth LL, et al. Lorazepam for seizure prophylaxis during high- dose busulfan administration. Bone Marrow Transplantation. 2002;29:963–965. doi: 10.1038/sj.bmt.1703593. [DOI] [PubMed] [Google Scholar]
- 48.Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulfan kinetics. Cancer Chemother Pharmacol. 1993;33:181–186. doi: 10.1007/BF00686213. [DOI] [PubMed] [Google Scholar]
- 49.De La Camara R, Tomas JF, Figuera A, Berberana M, Fernandez-Ranada JM. High-dose busulfan and seizures. Bone Marrow Transplant. 1991;7:363–364. [PubMed] [Google Scholar]
- 50.Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. doi: 10.1007/s002800050404. [DOI] [PubMed] [Google Scholar]
- 51.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplant conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 52.Hassan M, Ljungman P, Bolme P, et al. Busulfan bioavailability. Blood. 1994;84:2144–2150. [PubMed] [Google Scholar]
- 53.Schuler US, Ehrsam M, Schneider A, Schmidt H, Deeg J, Ehninger G. Pharmacokinetics of intravenous busulfan and evaluation of the bioavailability of the oral formulation in conditioning for haematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;22:241–244. doi: 10.1038/sj.bmt.1701322. [DOI] [PubMed] [Google Scholar]
- 54.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 55.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 56.Thall PF, Champlin RE, Andersson BS. Comparisson of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant. 2004;33:1191–1199. doi: 10.1038/sj.bmt.1704461. [DOI] [PubMed] [Google Scholar]
- 57.Kim SE, Lee JH, Choi SJ, Lee JH, Ryu SG, Lee KH. Morbidity and non-relapse mortality after allogeneic bone marrow transplantation in adult leukemia patients conditioned with busulfan plus cyclophosphamide: a retrospective comparison of oral versus intravenous busulfan. Haematologica. 2005;90:285–286. [PubMed] [Google Scholar]
- 58.Lee JH, Choi SJ, Lee JH, et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321–330. doi: 10.1007/s00277-004-0982-4. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal C, Gupta S, Vaughan WP, et al. Improved outcomes in intermediate- and high-risk aggressive non-Hodgkin lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral busulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biol Blood Marrow Transplant. 2006;12:77–777. doi: 10.1016/j.bbmt.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Dean R, Pohlman B, Sweetenham JW, Sobecks R, et al. The use of intravenous compared with oral busulfan in high-dose chemotherapy and autologous stem cell transplantation (ASCT) for lymphoma is associated with improved relapse-free survival. Blood. 2006;108:868a. [Google Scholar]
- 61.DeLeve LD, Schulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 62.Ghandi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Liu X, Glassman AB, Keating MJ, Stros M, Plunkett W, et al. Fludarabine triphosphate inhibits nucleotide excision repair of cisplatin-induced DNA adducts in vitro. Cancer Res. 1997;57:1487–1494. [PubMed] [Google Scholar]
- 64.Terenzi A, Aristei C, Aversa F, et al. Efficacy of fludarabine as an immunosupressor for bone marrow transplantation conditioning: Preliminary results. Transplant Proc. 1996;28:3101. [PubMed] [Google Scholar]
- 65.Slavin S, Nagler A, Naperstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 66.Bornhauser M, Thiede C, Plazbecker U, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res. 2001;7:2254–2262. [PubMed] [Google Scholar]
- 67.Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 68.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–477. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 69.De Lima M, Couriel Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 70.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 71.Andersson BS, de Lima M, Thall PF, et al. Once daily IV busulfan and fludarabine compares favorably with IV busulfan and cyclophosphamide (IV BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chae YS, Sohn SK, Kim JG, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant. 2007;40:541–547. doi: 10.1038/sj.bmt.1705770. [DOI] [PubMed] [Google Scholar]
- 73.Bredeson CN, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meresse V, Hartmann O, Vassal G, et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992;10:135–141. [PubMed] [Google Scholar]
- 75.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relationship to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 76.Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G. High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant. 1997;20:909–913. doi: 10.1038/sj.bmt.1700994. [DOI] [PubMed] [Google Scholar]
- 77.Schuler U, Schroer S, Kuhnle A, et al. Busulfan pharmacokinetics in bone marrow transplant patients: is drug monitor warranted? Bone Marrow Transplant. 1994;14:759–765. [PubMed] [Google Scholar]
- 78.Vaughan WP, Cagnoni P, Fernandez H, Hu W, Kashyap A, Gian V, Wingard J, Tarantolo S, Andersson BS. Decreased incidence of and risk factors for hepatic veno- occlusive disease with an intravenous busulfan (BU) containing preparative regimen for hematopoietic stem cell transplantation (HSCT) Blood. 1998;92(Suppl. 1):516a. [Google Scholar]
- 79.De Leve L. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837. doi: 10.1002/hep.510240414. [DOI] [PubMed] [Google Scholar]
- 80.Johnson L, Orchard PJ, Baker KS, et al. Glutathione S-transferase A1 genetic variants reduce busulfan clearance in children undergoing hematopoietic cell transplantation. J Clin Pharmacol. 2008;48:1052–1062. doi: 10.1177/0091270008321940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 82.Deeg HJ, Shulman HM, Anderson JE, et al. Allogeneic and syngeneic marrow transplantation for myelodysplastic syndrome in patients 55 to 66 years of age. Blood. 2000;95:1188–1194. [PubMed] [Google Scholar]
- 83.Hassan M. Busulphan. In: Grochow LB, Ames NM, editors. A Clinician’s Guide to Chemotherapy, Pharmacokinetics, and Pharmacodynamics. Williams and Wilkins; Baltimore, MD: 1997. pp. 189–208. New York, NY. [Google Scholar]
- 84.Bollinger AM, Zangwill AB, Slattery JT, et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant. 001;28:1013–1018. doi: 10.1038/sj.bmt.1703264. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C. I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33:979–987. doi: 10.1038/sj.bmt.1704446. [DOI] [PubMed] [Google Scholar]
- 86.McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 87.Tran H, Petropoulos D, Worth L, Mullen CA, Madden T, Andersson BS. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Biol Blood Marrow Transplant. 2004;10:805–812. [Google Scholar]
- 88.Benet LZ, Sheiner LB. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. In: Goodman Gilman A, Goodman LS, Rall TW, Murad F., editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 7th MacMillan Publishing Co.; New York, NY: 1985. p. 8. [Google Scholar]
- 89.Geddes M, Kangarloo BS, Naveed F, et al. High busulfan exposure is associated with worse outcomes in daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 90.Nath CE, Earl JW, Pati N, Stephen K, Shaw PJ. Variability in the pharmacokinetics of intravenous busulphan given as a single daily dose to paediatric blood or marrow transplant recipients. Br J Clin Pharmacol. 2008;66:50–59. doi: 10.1111/j.1365-2125.2008.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hassan Z, Ljungman P, Ringden O, et al. Pharmacokinetics of liposomal busulphan in man. Bone Marrow Transplant. 2001;27:479–85. doi: 10.1038/sj.bmt.1702823. [DOI] [PubMed] [Google Scholar]
- 92.Hassan M, Nilsson C, Hassan Z, et al. A Phase II trial of liposomal busulphan as an intravenous myeloablative agent prior to stem cell transplantation: 500 mg/m2 as a optimal total dose for conditioning. Bone Marrow Transplant. 2002;30:833–41. doi: 10.1038/sj.bmt.1703739. [DOI] [PubMed] [Google Scholar]
- 93.Vassal G, Michel G, Esperou H, et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol. 2008;61:113–123. doi: 10.1007/s00280-007-0455-2. [DOI] [PubMed] [Google Scholar]
- 94.Hobbs JR, Hugh-Jones K, Shaw PJ, Downie CJ, Williamson S. Engraftment rates related to busulphan and cyclophosphamide dosages for displacement bone marrow transplants in fifty children. Bone Marrow Transplant. 1986;1:201–208. [PubMed] [Google Scholar]
- 95.Lucarelli G, Polchi P, Galimberti M, et al. Marrow transplantation for thalassaemia following busulphan and cyclophosphamide. Lancet. 1985;(8442):1, 1355–1357. doi: 10.1016/s0140-6736(85)91784-2. [DOI] [PubMed] [Google Scholar]
- 96.Grochow LB, Krivit W, Whitley CB, Blazar B. Busulfan disposition in children. Blood. 1990;75:1723–1727. [PubMed] [Google Scholar]
- 97.Yaeger AM, Wagner JE, Graham ML, Jones RJ, Santos GW, Grochow LB. Optimization of busulfan dosage in children undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood. 1992;80:2425–2428. [PubMed] [Google Scholar]
- 98.Hassan M, Oberg G, Bekassy AN, et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol. 1991;28:130–134. doi: 10.1007/BF00689702. [DOI] [PubMed] [Google Scholar]
- 99.Vassal G, Deroussent A, Challine D, et al. Is 600 mg/m2 the appropriate dosage of busulfan in children undergoing bone marrow transplantation? Blood. 1992;79:2475–2479. [PubMed] [Google Scholar]
- 100.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999;27:1466–1469. [PubMed] [Google Scholar]
- 101.Shaw PJ, Scharping CE, Brian RJ, Earl JW. Busulfan pharmacokinetics using a single daily high-dose regimen in children with acute leukemia. Blood. 1994;84:2357–2362. [PubMed] [Google Scholar]
- 102.Vassal G, Koscielny S, Challine D, et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1996;37:247–253. doi: 10.1007/BF00688324. [DOI] [PubMed] [Google Scholar]
- 103.Lindley C, Shea T, McCune J, et al. Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy. Anticancer Drugs. 2004;20:909–913. doi: 10.1097/01.cad.0000127145.50172.51. [DOI] [PubMed] [Google Scholar]
- 104.Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18:340–47. doi: 10.1200/JCO.2000.18.2.340. [DOI] [PubMed] [Google Scholar]
- 105.Worth LL, Andersson B, Papadoupolos D, et al. Long-term follow-up of children with acute leukemia conditioned with Thiotepa, individualized busulfan, and cyclophosphamide (TBC) with allogeneic stem cell transplantation. (Submitted, 2008/ In press, 2009) [Google Scholar]
- 106.Russell JA, Savoie ML, Balogh A, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 cGy total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Bishop MR, Logan BR, Gandham S, et al. Long-term outcomes of adults with acute lymphoblastic leukemia after autologous or unrelated donor bone marrow transplantation: a comparative analysis by the National Marrow Donor Program and Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;41:635–642. doi: 10.1038/sj.bmt.1705952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horwitz ME, Morris A, Gasparetto C, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant. 2008;14:591–594. doi: 10.1016/j.bbmt.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanz J, Arcese W, Hernandez-Boluda JV, et al. Low transplant-related mortality in patients with hematologic malignancies undergoing umbilical cord blood transplantation (UCBT) with thiotepa (TT), once-daily intravenous (IV) busulfan (BU), fludarabine (FLU) and anti-thymocyte globulin (ATG) Blood. 2007;110:618a. [Google Scholar]
- 110.Srivastava A, Bradstock KF, Szer J, de Bortoli L, Gottlieb DJ. Busulphan and melphalan prior to autologous bone marrow transplantation. Bone Marrow Transplant. 1993;12:323–329. [PubMed] [Google Scholar]
- 111.Martino R, Badell I, Brunet S, et al. High-dose busulfan and melphalan before bone marrow transplantation for acute nonlymphoblastic leukemia. Bone Marrow Transplant. 1995;16:209–212. [PubMed] [Google Scholar]
- 112.Vey N, De Prijck B, Faucher C, et al. A pilot study of busulfan and melphalan as preparatory regimen prior to allogeneic bone marrow transplantation in refractory or relapsed hematological malignancies. Bone Marrow Transplant. 1996;18:495–499. [PubMed] [Google Scholar]
- 113.Matsuyama T, Kojima S, Kato K. Allogeneic bone marrow transplantation for childhood leukemia following a busulfan and melphalan preparative regimen. Bone Marrow Transplant. 1998;22:21–26. doi: 10.1038/sj.bmt.1701276. [DOI] [PubMed] [Google Scholar]
- 114.Small TN, Young JW, Castro-Malaspina H, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA- matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biol Blood Marrow Transplant. 2007;13:235–244. doi: 10.1016/j.bbmt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Kebriaei P, Giralt S, Madden TL, et al. Toxicity and early response after intravenous (IV) busulfan (Bu) plus melphalan (Mel) conditioning for autologous stem cell transplantation (SCT) in patients (pts) with multiple myeloma (MM) Blood. 2006;108:2943a. [Google Scholar]
- 116.Wall DA, Carter SL, Kernan NA, et al. Busulfan/Melphalan/antithymocyte globulin followed by unrelated donor cord blood transplantation for treatment of infant leukemia and leukemia in young children: the Cord Blood Transplantation study (COBLT) experience. Biol Blood Marrow Transplant. 2005;11:637–46. doi: 10.1016/j.bbmt.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 117.Pui CH, Jeha S. Clofarabine. Nat Rev Drug Discov. 2005:S12–13. doi: 10.1038/nrd1724. [DOI] [PubMed] [Google Scholar]
- 118.Cohen MH, Johnson JR, Justice R, Pazdur R. FDA drug approval summary: nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008;13:709–714. doi: 10.1634/theoncologist.2006-0017. [DOI] [PubMed] [Google Scholar]
- 119.Andersson BS, de Lima M, Worth LL, et al. Clofarabine (Clo) +/− fludarabine (Flu) with IV busulfan (Bu) and allogeneic stem cell transplantation (Allo-SCT) for relapsed, refractory myeloid leukemia and MDS. Blood. 2008 (abstract submitted) [Google Scholar]