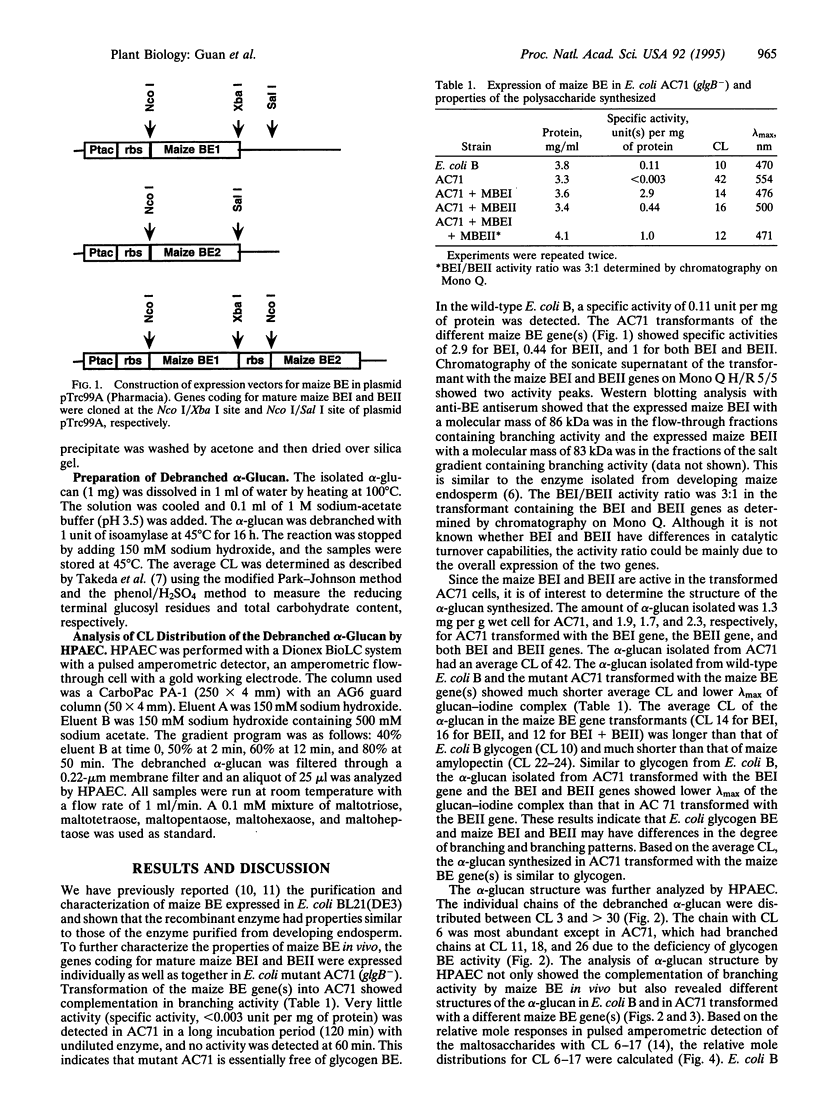
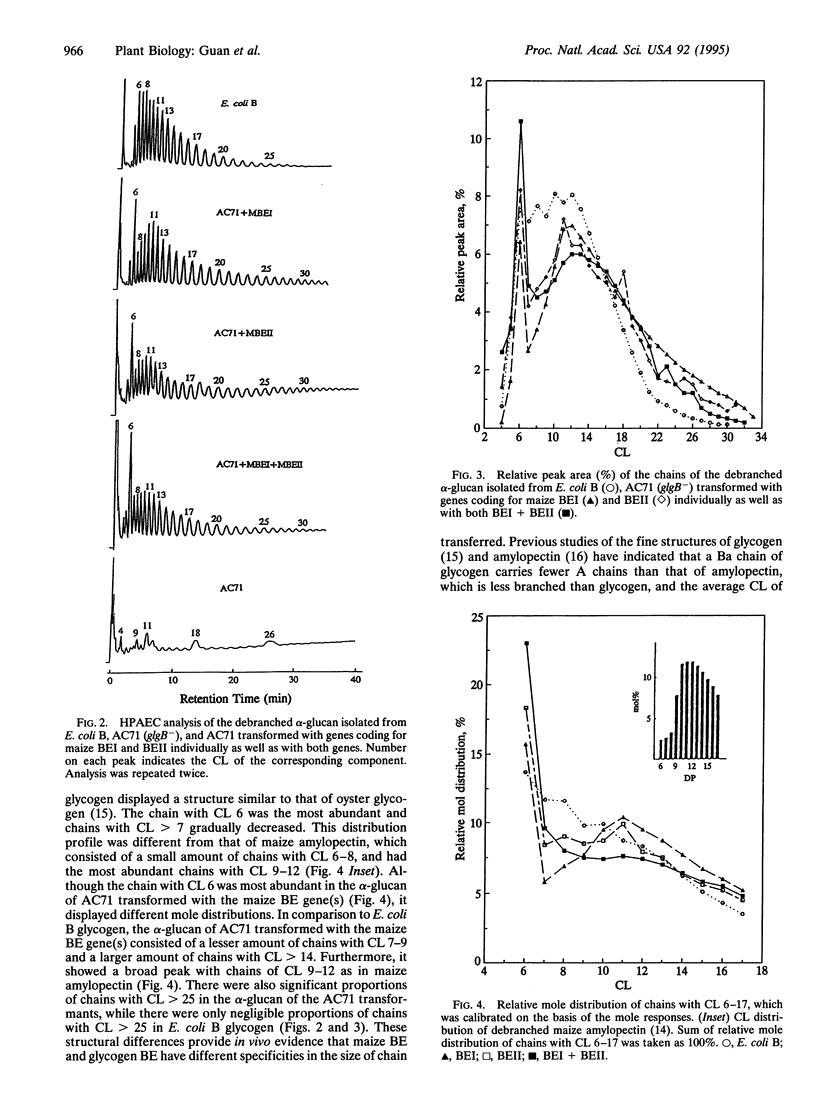
Abstract
The structure of alpha-glucan, isolated from wild-type Escherichia coli B, a glycogen branching enzyme (BE)-deficient E. coli AC71 (glgB-), or from AC71 transformed with genes coding for maize BEI and BEII individually as well as with both genes, was analyzed by high-performance anion-exchange chromatography (HPAEC) with pulsed amperometric detection. Transformation of the maize BE gene(s) in AC71 (glgB-) showed complementation in branching activity. Analysis by HPAEC revealed different structures between glycogen of E. coli B and alpha-glucan of AC71 transformed with a different maize BE gene(s). The individual chains of the alpha-glucan debranched with isoamylase were distributed between chain length (CL) 3 and > 30 and the chain with CL 6 was the most abundant. In comparison with the glycogen of E. coli B, the alpha-glucan of AC71 transformed with the maize BE gene(s) consisted of a lesser amount of chains with CL 7-9 and a larger amount of chains with CL > 14. It also showed a broad peak with chains of CL 9-12 as in maize amylopectin. This study provides in vivo evidence that glycogen BE and maize BE isozymes may have different specificities in the length of chain transferred. Furthermore, this study suggests that the specificity of glycogen synthase and starch synthase and their concerted action with BE play an important role in determining the structure of the polysaccharide synthesized.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba T., Kimura K., Mizuno K., Etoh H., Ishida Y., Shida O., Arai Y. Sequence conservation of the catalytic regions of amylolytic enzymes in maize branching enzyme-I. Biochem Biophys Res Commun. 1991 Nov 27;181(1):87–94. doi: 10.1016/s0006-291x(05)81385-3. [DOI] [PubMed] [Google Scholar]
- Fisher D. K., Boyer C. D., Hannah L. C. Starch branching enzyme II from maize endosperm. Plant Physiol. 1993 Jul;102(3):1045–1046. doi: 10.1104/pp.102.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T., D'Hulst C., Maddelein M. L., Routier F., Pépin T. M., Decq A., Wieruszeski J. M., Delrue B., Van den Koornhuyse N., Bossu J. P. Toward an understanding of the biogenesis of the starch granule. Evidence that Chlamydomonas soluble starch synthase II controls the synthesis of intermediate size glucans of amylopectin. J Biol Chem. 1993 Aug 5;268(22):16223–16230. [PubMed] [Google Scholar]
- Guan H. P., Baba T., Preiss J. Expression of branching enzyme I of maize endosperm in Escherichia coli. Plant Physiol. 1994 Apr;104(4):1449–1453. doi: 10.1104/pp.104.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. P., Baba T., Preiss J. Expression of branching enzyme II of maize endosperm in Escherichia coli. Cell Mol Biol (Noisy-le-grand) 1994 Nov;40(7):981–988. [PubMed] [Google Scholar]
- Guan H. P., Preiss J. Differentiation of the Properties of the Branching Isozymes from Maize (Zea mays). Plant Physiol. 1993 Aug;102(4):1269–1273. doi: 10.1104/pp.102.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Mercier C., Smith E. E., Whelan W. J. A revision of the Meyer-Bernfeld model of glycogen and amylopectin. FEBS Lett. 1970 Dec 28;12(2):101–104. doi: 10.1016/0014-5793(70)80573-7. [DOI] [PubMed] [Google Scholar]
- Preiss J., Greenberg E., Sabraw A. Biosynthesis of bacterial glycogen. Kinetic studies of a glucose-1-phosphate adenylyltransferase (EC 2.7.7.27) from a glycogen-deficient mutant of Escherichia coli B. J Biol Chem. 1975 Oct 10;250(19):7631–7638. [PubMed] [Google Scholar]
- Preiss J., Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–238. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- Shewmaker C. K., Boyer C. D., Wiesenborn D. P., Thompson D. B., Boersig M. R., Oakes J. V., Stalker D. M. Expression of Escherichia coli glycogen synthase in the tubers of transgenic potatoes (Solanum tuberosum) results in a highly branched starch. Plant Physiol. 1994 Apr;104(4):1159–1166. doi: 10.1104/pp.104.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]



