Abstract
A particulate preparation (MgP) capable of photosynthetic CO2 assimilation without the addition of stromal protein was obtained by rupturing whole spinach (Spinacia oleracea var. America) chloroplasts in 15 millimolar MgCl2 buffered with Tricine at pH 8.5. This CO2 assimilation was dependent upon light, inorganic phosphate, ferredoxin, ADP, NAD or NADP, and primer. Excepting glycolate, the products of CO2 fixation by MgP were similar to those found with whole chloroplasts.
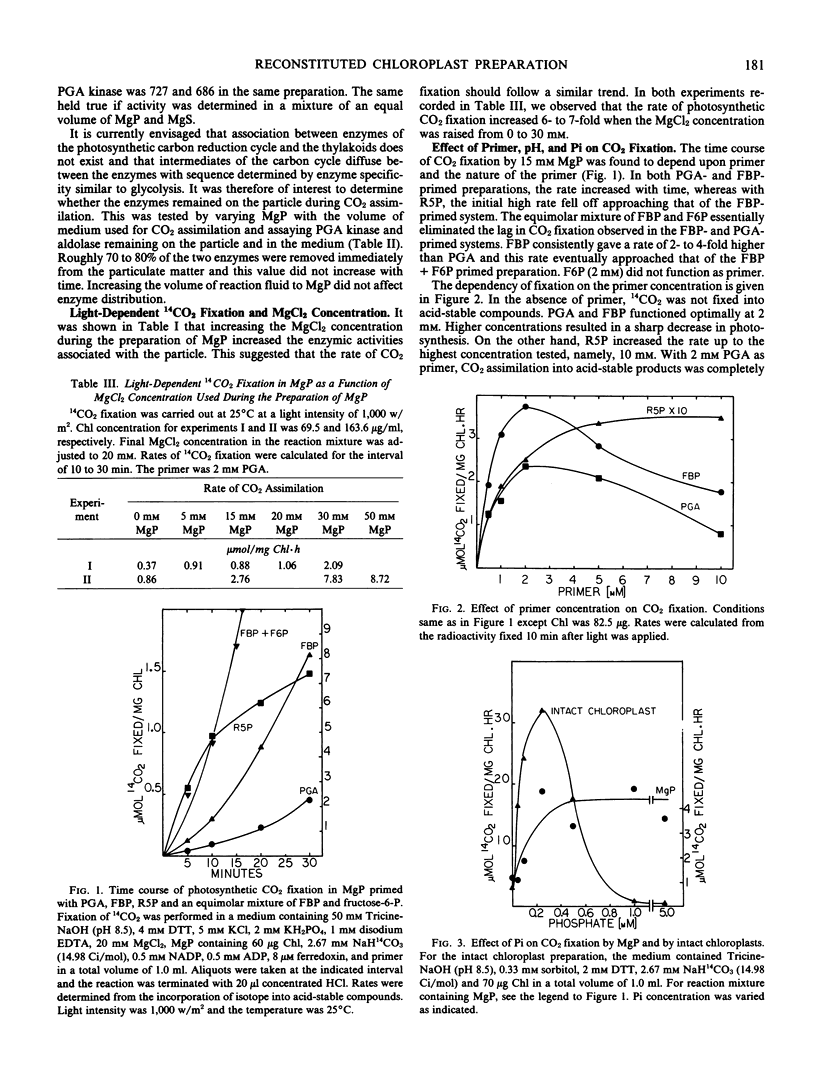
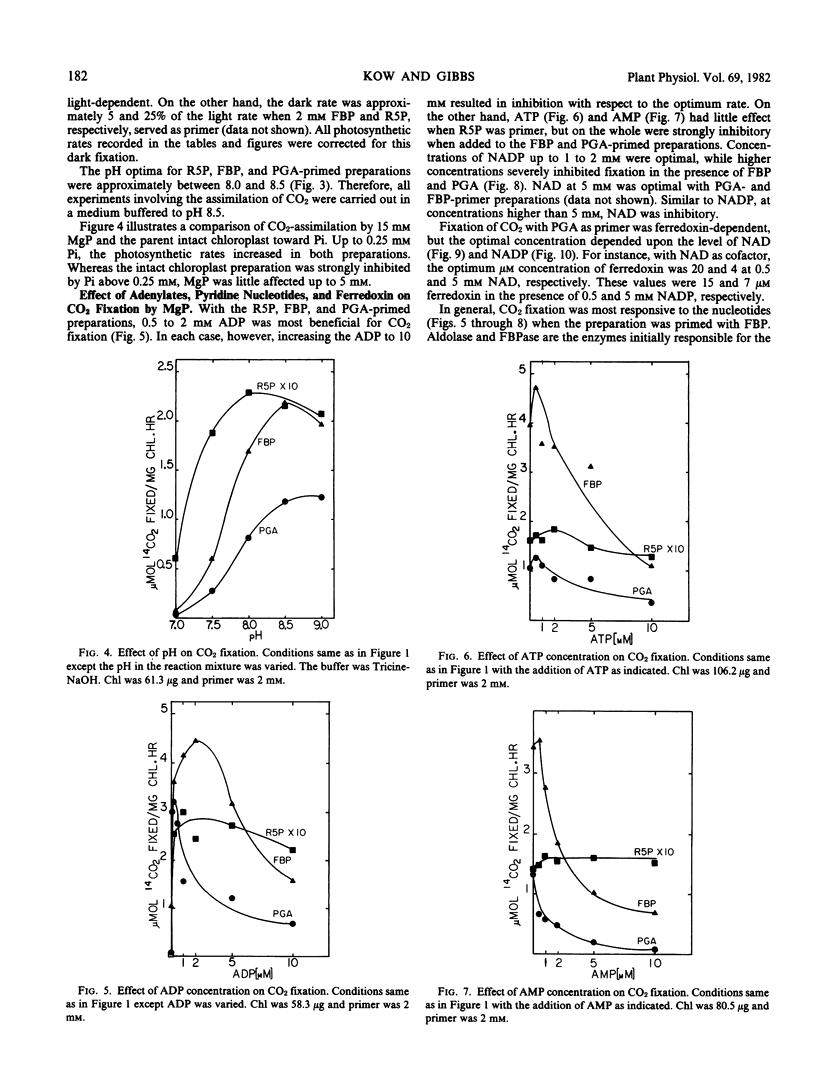
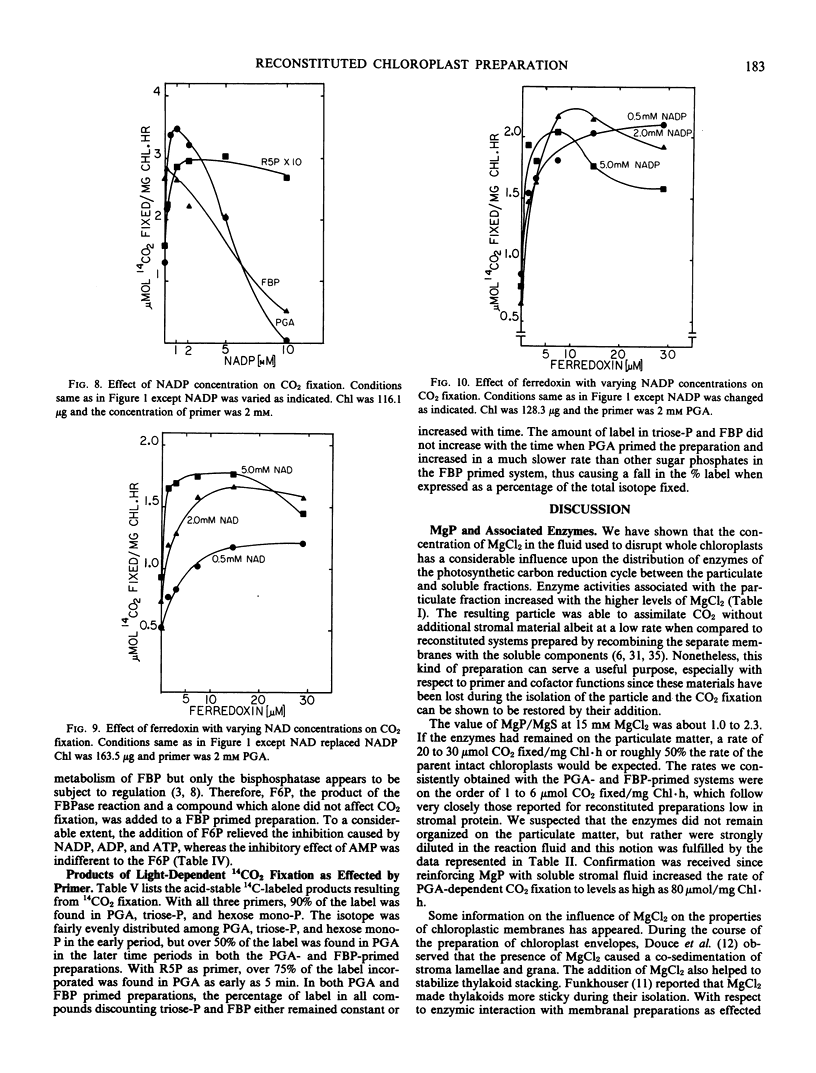
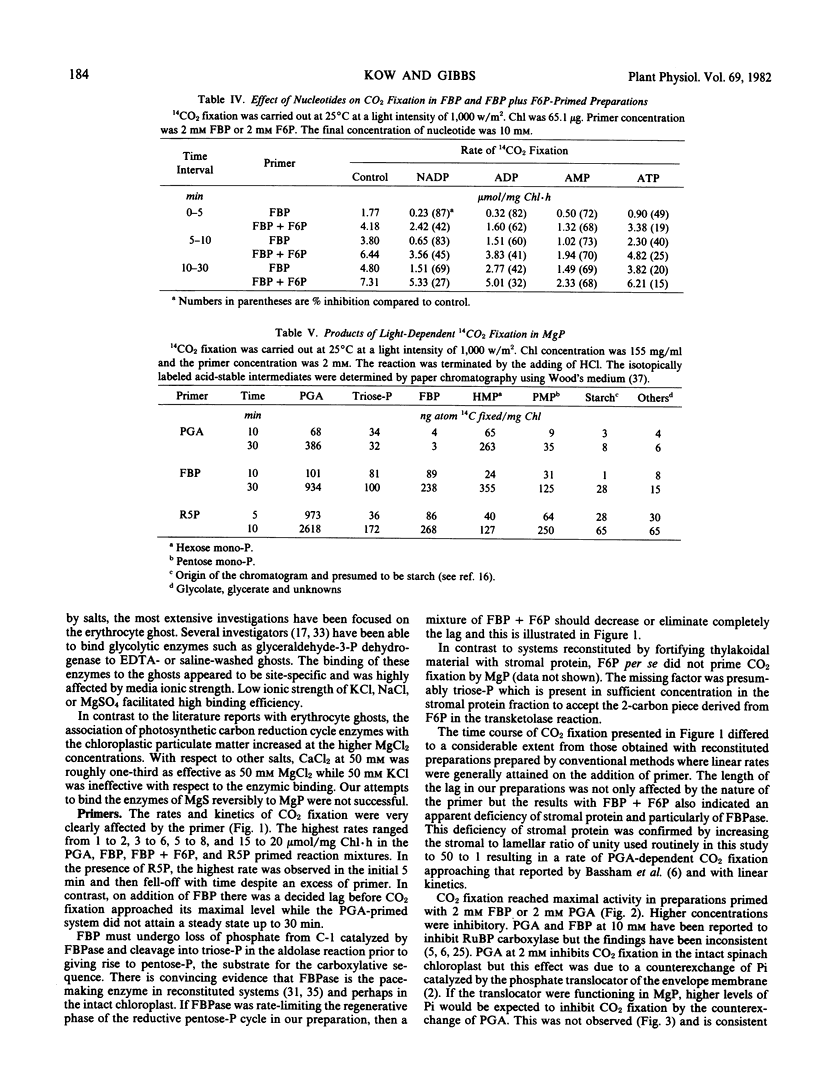
Glycerate-3-phosphate (PGA), fructose-1, 6-bisphosphate (FBP), and ribose-5-phosphate (R5P) but not fructose-6-P (F6P) functioned as primers. Concentrations of PGA and FBP but not of R5P higher than 2 millimolar were inhibitory to CO2 fixation. A lag of CO2 fixation was observed with PGA and FBP but not with R5P. This lag as well as inhibition by NADP, ADP, and ATP in the FBP-primed preparation was eliminated by an equimolar mixture of FBP plus F6P indicating FBPase as the sensitive site. NADP, ADP, and ATP also blocked CO2 fixation by the PGA-fortified preparation but inhibition was even more sensitive than that observed when FBP was added. Inhibition by AMP in the PGA and FBP-primed preparations was not affected by the addition of F6P. When R5P was the starting primer, inhibition of CO2 fixation was relatively insensitive to the adenylates and NADP. In contrast to the parent whole chloroplast, CO2 fixation by MgP was insensitive to high (5 millimolar) inorganic phosphate. Depending upon the ferredoxin concentration, NAD was as effective as NADP in supporting CO2 fixation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen W. R., Gibbs M. Inhibition of CO2 fixation in intact spinach chloroplasts by 3-phosphoglyceric acid. Biochem Biophys Res Commun. 1975 Feb 17;62(4):953–956. doi: 10.1016/0006-291x(75)90415-5. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Regulation of pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta. 1973 Oct 10;321(2):484–488. doi: 10.1016/0005-2744(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Kalberer P. P., Arnon D. I. Ferredoxin-activated fructose diphosphatase in isolated chloroplasts. Biochem Biophys Res Commun. 1967 Oct 11;29(1):74–79. doi: 10.1016/0006-291x(67)90543-8. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P. Regulation of ribulose 1,5-diphosphate carboxylase in the photosynthetic assimilation of carbon dioxide. J Biol Chem. 1973 Jul 25;248(14):4956–4964. [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Regulation of ribulose 1,5-diphosphate carboxylase by substrates and other metabolites: further evidence for several types of binding sites. Plant Physiol. 1975 Apr;55(4):720–726. doi: 10.1104/pp.55.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- HAVIR E. A., GIBBS M. STUDIES ON THE REDUCTIVE PENTOSE PHOSPHATE CYCLE IN INTACT AND RECONSTITUTED CHLOROPLAST SYSTEMS. J Biol Chem. 1963 Oct;238:3183–3187. [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Higashi T., Richards C. S., Uyeda K. The interaction of phosphofructokinase with erythrocyte membranes. J Biol Chem. 1979 Oct 10;254(19):9542–9550. [PubMed] [Google Scholar]
- Johnson E. J. Occurrence of the adenosine monophosphate inhibition of carbon dioxide fixation in photosynthetic and chemosynthetic autotrophs. Arch Biochem Biophys. 1966 Apr;114(1):178–183. doi: 10.1016/0003-9861(66)90319-5. [DOI] [PubMed] [Google Scholar]
- LOSADA M., TREBST A. V., ARNON D. I. Photosynthesis by isolated chloroplasts. XI. Carbon dioxide assimilation in a reconstituted chloroplast system. J Biol Chem. 1960 Mar;235:832–839. [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- Lendzian K., Bassham J. A. NADPH/NADP+ ratios in photosynthesizing reconstituted chloroplasts. Biochim Biophys Acta. 1976 Jun 8;430(3):478–489. doi: 10.1016/0005-2728(76)90024-4. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Heldt H. W. The activation of ribulose 1,5-bisphosphate carboxylase/oxygenase. Basic Life Sci. 1978;11:283–306. doi: 10.1007/978-1-4684-8106-8_18. [DOI] [PubMed] [Google Scholar]
- McNeil P. H., Walker D. A. The effect of magnesium and other ions on the distribution of ribulose 1,5-bisphosphate carboxylase in chloroplast extracts. Arch Biochem Biophys. 1981 Apr 15;208(1):184–188. doi: 10.1016/0003-9861(81)90138-7. [DOI] [PubMed] [Google Scholar]
- Nelson N., Neumann J. Interaction between ferredoxin and ferredoxin nicotinamide adenine dinucleotide phosphate reductase in pyridine nucleotide photoreduction and some partial reactions. I. Inhibition of ferredoxin nicotinamide adenine dinucleotide phosphate reductase by ferredoxin. J Biol Chem. 1969 Apr 10;244(7):1926–1931. [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Chloroplast and Cytoplasmic Enzymes: VI. Pea Leaf 3-Phosphoglycerate Kinases. Plant Physiol. 1975 Feb;55(2):168–171. doi: 10.1104/pp.55.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAN PIETRO A., LANG H. M. Photosynthetic pyridine nucleotide reductase. I. Partial purification and properties of the enzyme from spinach. J Biol Chem. 1958 Mar;231(1):211–229. [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Transient inhibition by ribose 5-phosphate of photosynthetic O2 evolution in a reconstituted chloroplast system. Biochim Biophys Acta. 1976 Apr 9;430(1):154–164. doi: 10.1016/0005-2728(76)90231-0. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Phosphoglycerate as a hill oxidant in a reconstituted chloroplast system. Plant Physiol. 1971 Aug;48(2):163–165. doi: 10.1104/pp.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapazon E., Steck T. L. Interaction of the aldolase and the membrane of human erythrocytes. Biochemistry. 1977 Jun 28;16(13):2966–2971. doi: 10.1021/bi00632a025. [DOI] [PubMed] [Google Scholar]
- TREBST A. V., LOSADA M., ARNON D. I. Photosynthesis by isolated chloroplasts. XII. Inhibitors of carbon dioxide assimilation in a reconstituted chloroplast system. J Biol Chem. 1960 Mar;235:840–844. [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R., Fitzgerald M. P. Photosynthesis in a reconstituted chloroplast system from spinach. Some factors affecting CO2-dependent oxygen evolution with fructose-1,6-bisphosphate as substrate. Biochim Biophys Acta. 1976 Jul 9;440(1):147–162. doi: 10.1016/0005-2728(76)90120-1. [DOI] [PubMed] [Google Scholar]


