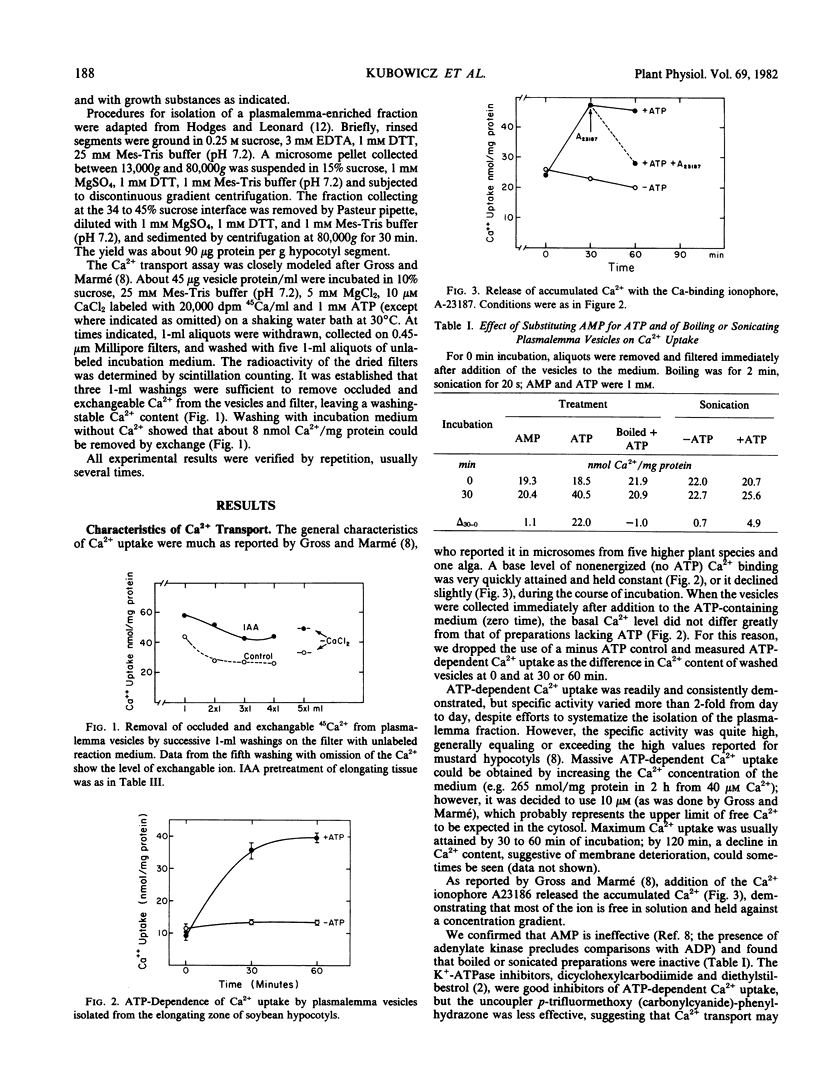
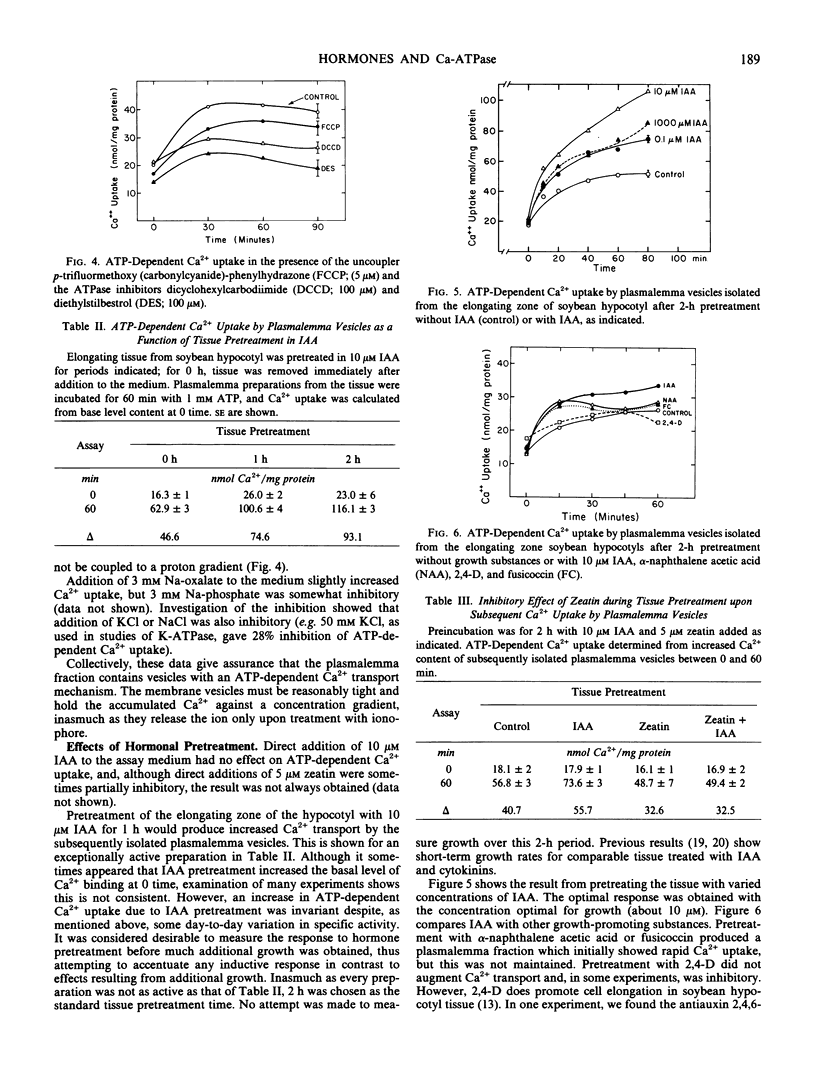
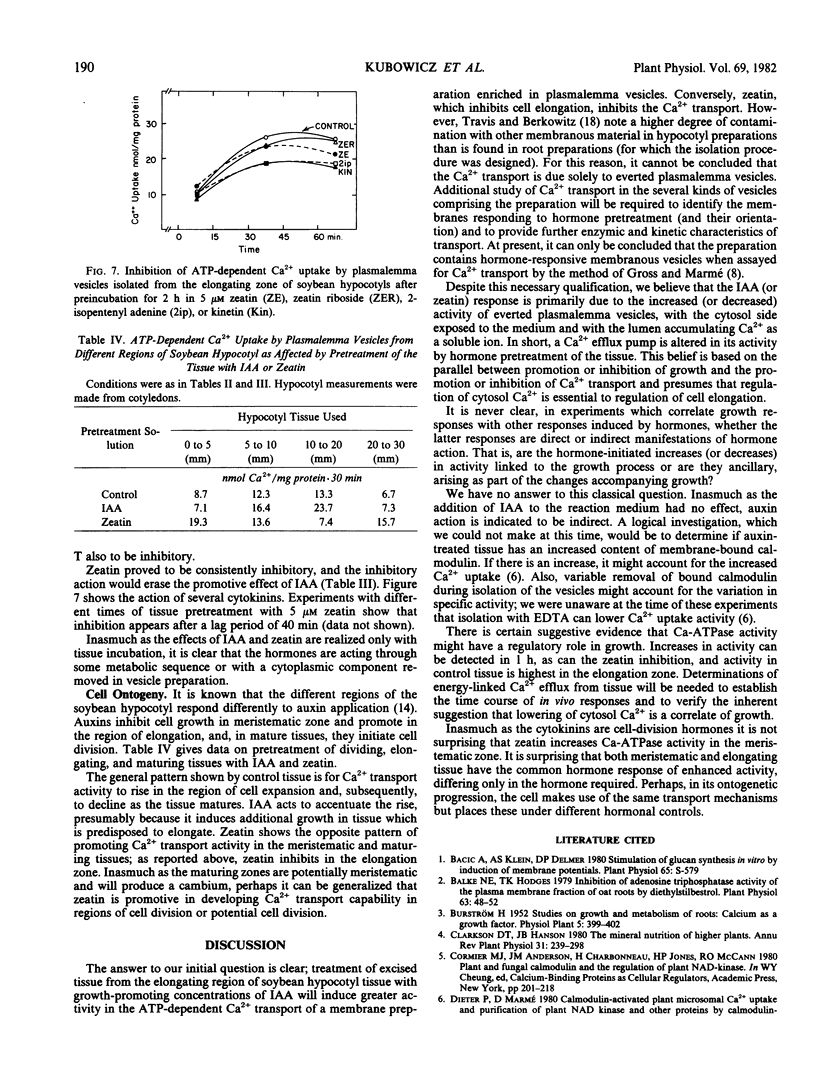
Abstract
A plasmalemma-enriched membrane preparation from etiolated soybean (Glycine max L., cv. Wayne) hypocotyls possesses an active ATP-dependent calcium pump which leads to calcium accumulation when assayed by the methods of Gross and Marmé (1978, Proc Natl Acad Sci USA 75: 1232-1236). Two-hour treatment of segments from the elongating zone of the hypocotyl with growth-promoting concentrations of indoleacetic acid gives up to 100 percent increase in the calcium transport activity. Conversely, similar pretreatment with zeatin or other cytokinins is inhibitory. In the meristematic and maturing zones of the hypocotyl, zeatin has the opposite effect of promoting calcium transport activity. One facet of cell-growth regulation may lie with hormonally mediated changes in efflux pumping of calcium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Hanson J. B. Some effects of 2,4-dichlorophenoxyacetic acid on soluble nucleotides & nucleic acid of soybean seedlings. Plant Physiol. 1961 Mar;36(2):145–152. doi: 10.1104/pp.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. Evolution and function of calcium-binding proteins. Int Rev Cytol. 1976;46:323–393. doi: 10.1016/s0074-7696(08)60994-8. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Berkowitz R. L. Characterization of Soybean Plasma Membrane during Development: FREE STEROL COMPOSITION AND CONCANAVALIN A BINDING STUDIES. Plant Physiol. 1980 May;65(5):871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]


