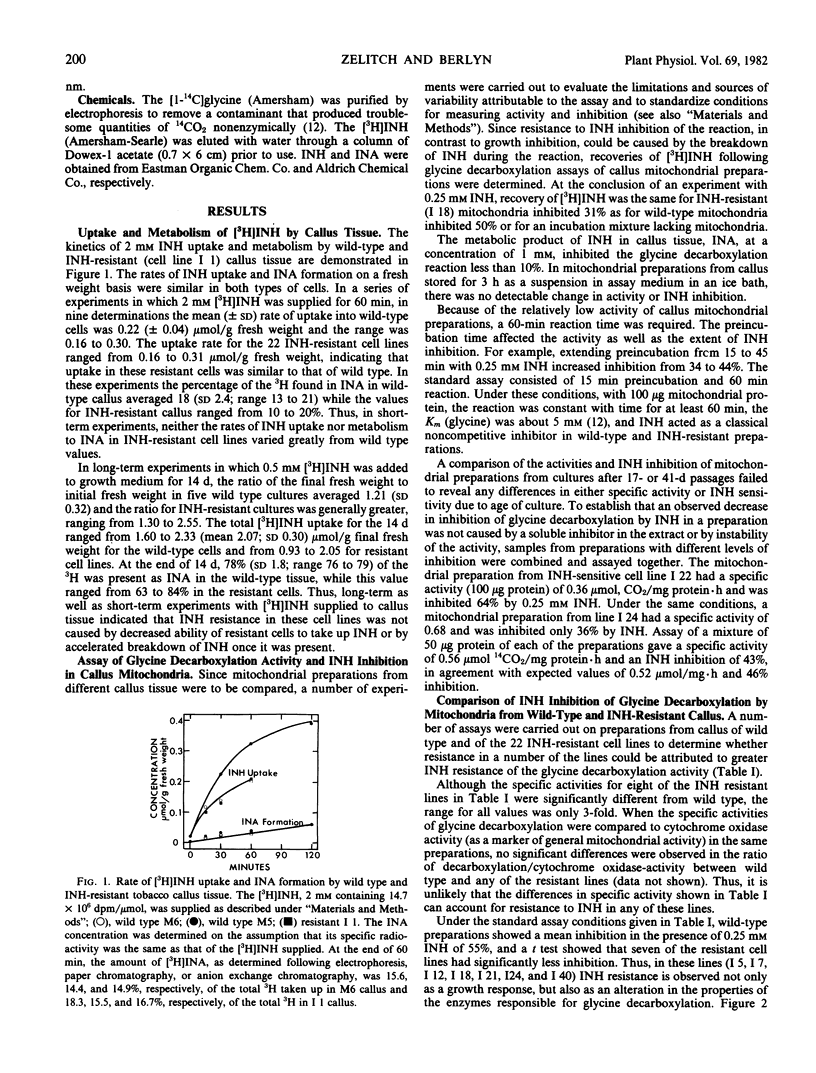
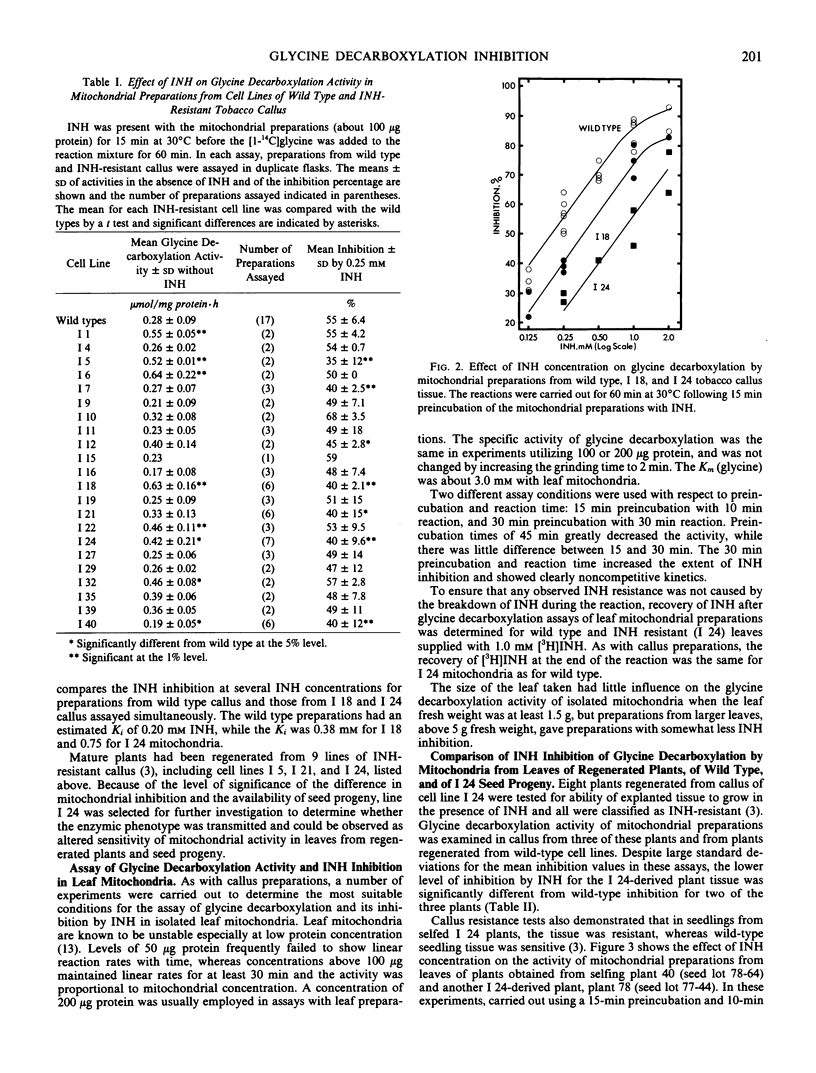
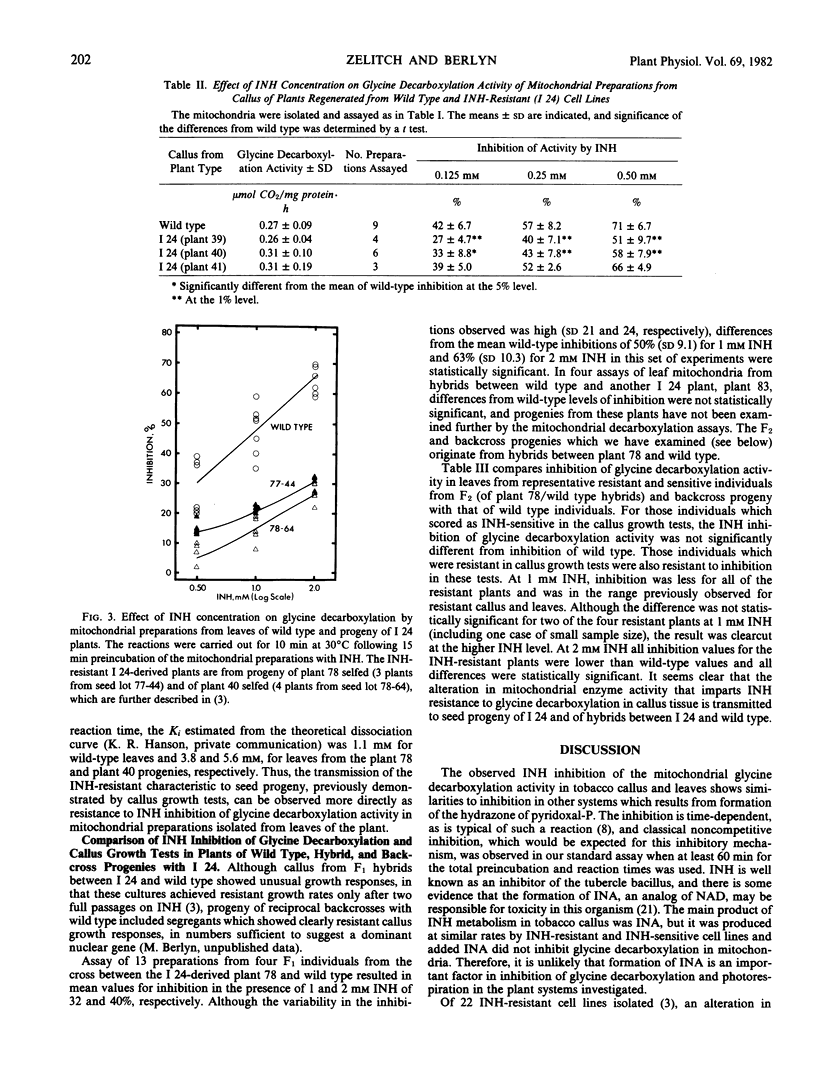
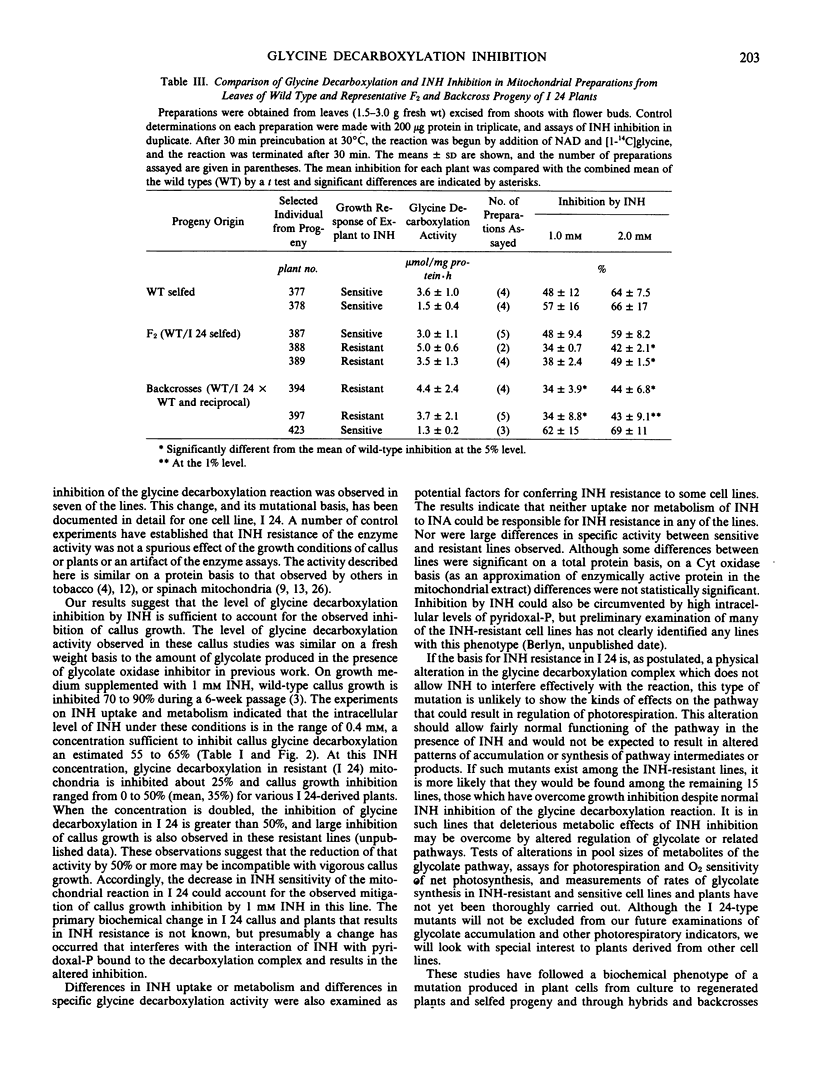
Abstract
Isonicotinic acid hydrazide (INH), an inhibitor of the photorespiratory pathway blocking the conversion of glycine to serine and CO2, has been used as a selective agent to obtain INH-resistant tobacco (Nicotiana tabacum) callus cells. Of 22 cell lines that were INH-resistant, none were different from wild-type cells in their ability to take up [3H]INH or to oxidize INH to isonicotinic acid. In 7 of the 22 cell lines, INH resistance was associated with decreased inhibition of NAD-dependent glycine decarboxylation activity in isolated mitochondrial preparations. In the cell line that was most extensively investigated (I 24), this biochemical phenotype (exhibiting a 3-fold higher Ki with INH) was observed in leaf mitochondria of regenerated plants and of plants produced from them by self-fertilization. After crosses between resistant and sensitive plants, the decreased inhibition of glycine decarboxylation was observed among F2 and backcross progeny only in those plants previously identified as INH-resistant by callus growth tests. In contrast, in siblings identified as INH-sensitive, glycine decarboxylation was inhibited by INH at the wild-type level. This demonstration of the transfer of an altered enzyme property from callus to regenerated plants and through seed progeny fulfills an important requirement for the use of somatic cell genetics to produce biochemical mutants of higher plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CUTHBERTSON W. F., IRELAND D. M., WOLFF W. Detection and identification of some metabolites of isonicotinic acid hydrazide (isoniazid) in human urine. Biochem J. 1953 Nov;55(4):669–671. doi: 10.1042/bj0550669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON A. N. The mechanism of the inhibition of decarboxylase by isonicotinyl hydrazide. Biochim Biophys Acta. 1956 Jan;19(1):131–140. doi: 10.1016/0006-3002(56)90394-8. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glutamate:glyoxylate aminotransferase activity in tobacco leaves and callus by glycidate, an inhibitor of photorespiration. Plant Physiol. 1978 Feb;61(2):242–247. doi: 10.1104/pp.61.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Oliver D. J. Effect of Glyoxylate on the Sensitivity of Net Photosynthesis to Oxygen (the Warburg Effect) in Tobacco. Plant Physiol. 1978 Dec;62(6):938–940. doi: 10.1104/pp.62.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J. Mechanism of decarboxylation of glycine and glycolate by isolated soybean cells. Plant Physiol. 1979 Dec;64(6):1048–1052. doi: 10.1104/pp.64.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J., Zelitch I. Increasing photosynthesis by inhibiting photorespiration with glyoxylate. Science. 1977 Jun 24;196(4297):1450–1451. doi: 10.1126/science.867040. [DOI] [PubMed] [Google Scholar]
- Oliver D. J., Zelitch I. Metabolic regulation of glycolate synthesis, photorespiration, and net photosynthesis in tobacco by L-glutamate. Plant Physiol. 1977 Apr;59(4):688–694. doi: 10.1104/pp.59.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiol. 1977 Oct;60(4):461–466. doi: 10.1104/pp.60.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel J. K., Schaper K. J., Wempe E., Cordes H. P. Mode of action and quantitative structure-activity correlations of tuberculostatic drugs of the isonicotinic acid hydrazide type. J Med Chem. 1976 Apr;19(4):483–492. doi: 10.1021/jm00226a007. [DOI] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C. Properties and intramitochondrial localization of serine hydroxymethyltransferase in leaves of higher plants. Plant Physiol. 1979 Apr;63(4):783–787. doi: 10.1104/pp.63.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Alternate pathways of glycolate synthesis in tobacco and maize leaves in relation to rates of photorespiration. Plant Physiol. 1973 Feb;51(2):299–305. doi: 10.1104/pp.51.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


