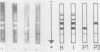Abstract
A ribonuclease fraction previously purified from flax by gel filtration was further resolved into two components by hydroxyl apatite chromatography. These were homogeneous with respect to electrophoresis and isoelectric focusing. Both enzymes are of RNase I type but differ in substrate specificity, kinetic properties, pH response, and isoelectric point.
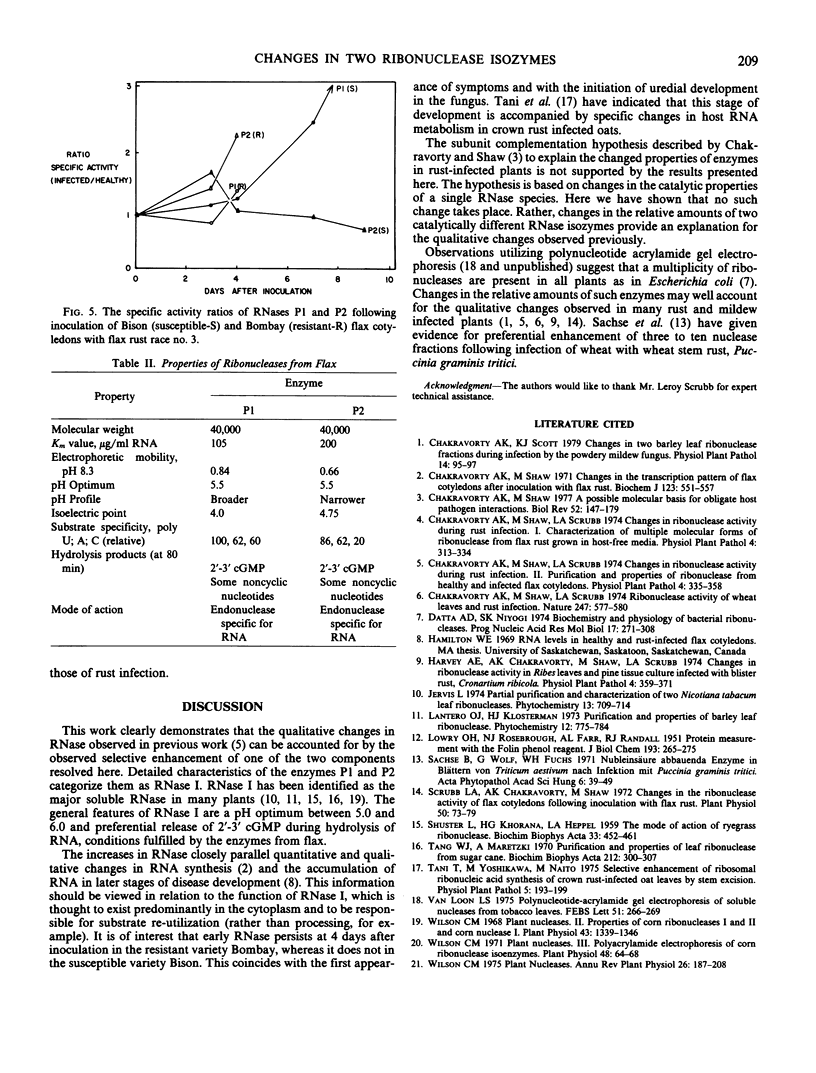
The two RNase isozymes show consistent properties when extracted from variety Bison (susceptible) or variety Bombay (resistant) with or without infection with race 3 of flax rust. The relative amounts of these isozymes change markedly during infection. These observations provide an explanation for the apparent qualitative changes in RNase noted previously. Differences between susceptible and resistant reactions in the early stages of disease are discussed.
Full text
PDF
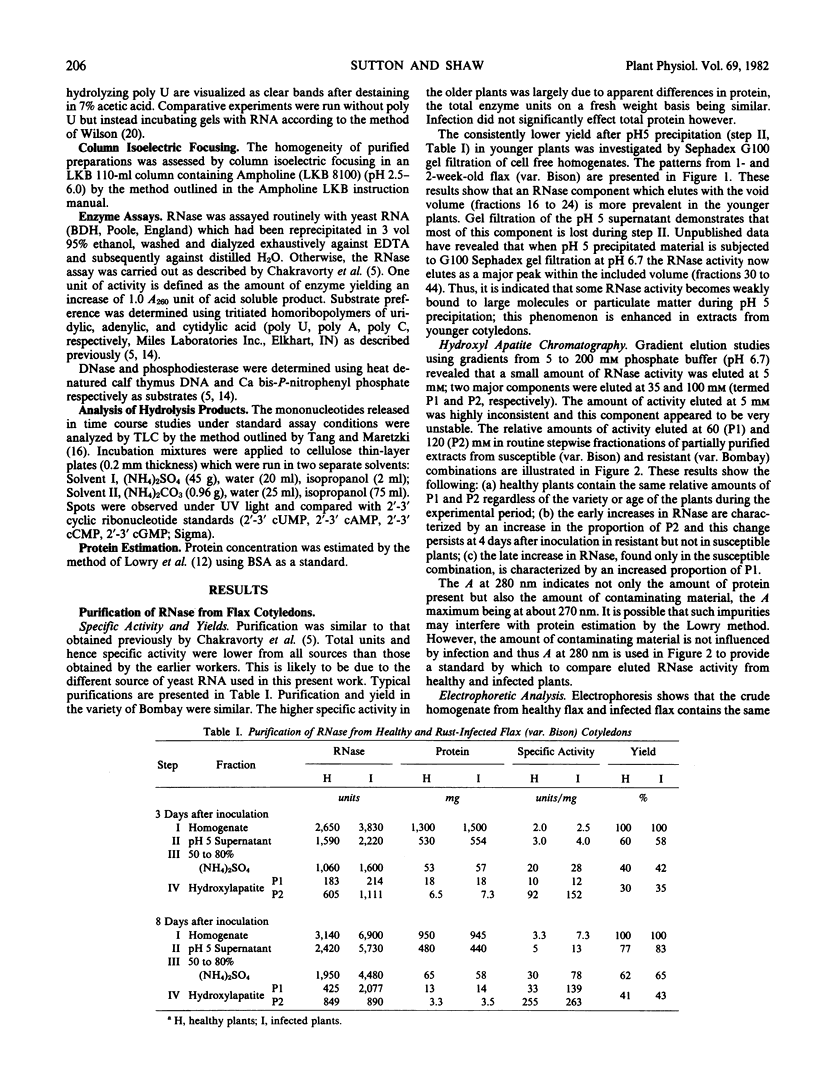
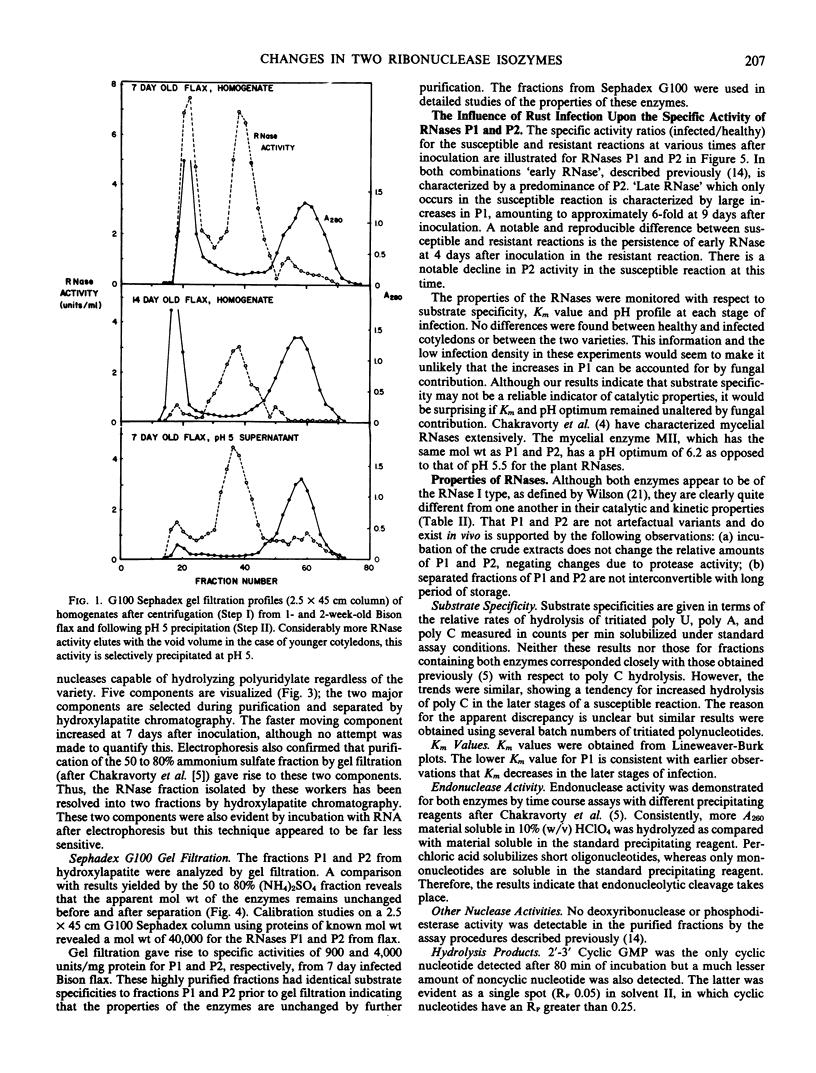
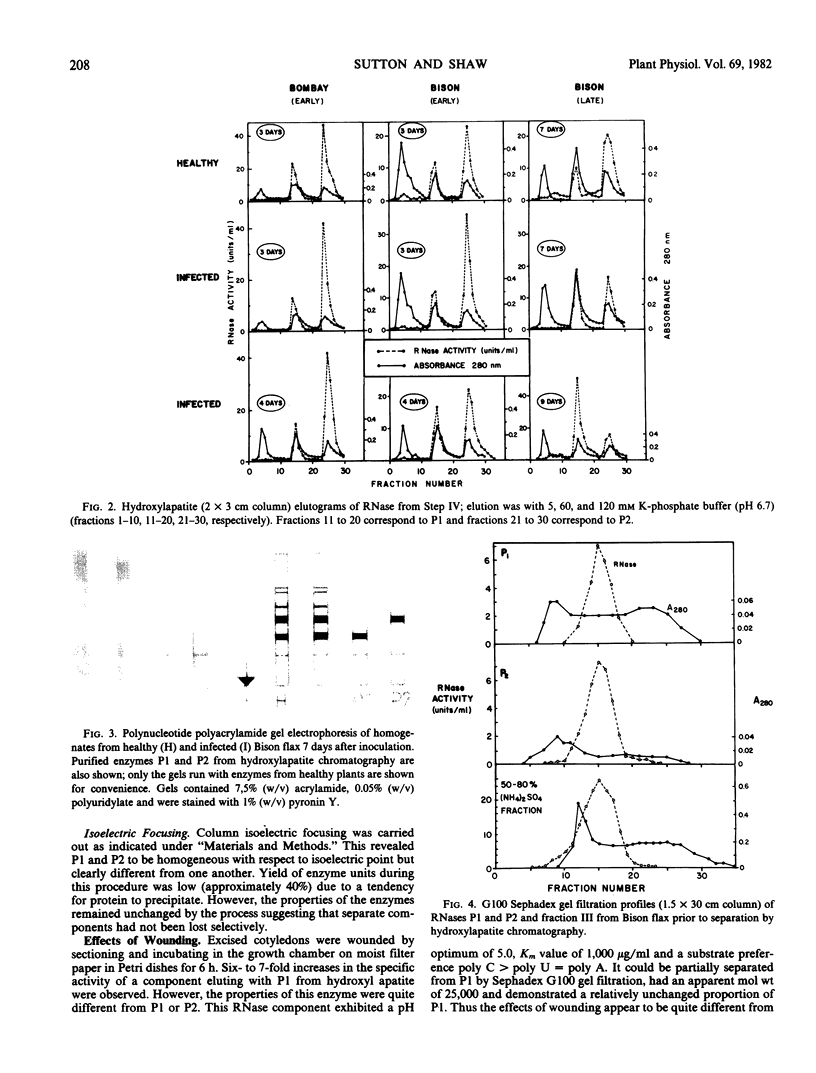
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakravorty A. K., Shaw M. A possible molecular basis for obligate host-pathogen interactions. Biol Rev Camb Philos Soc. 1977 May;52(2):147–179. doi: 10.1111/j.1469-185x.1977.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Chakravorty A. K., Shaw M. Changes in the transcription pattern of flax cotyledons after inoculation with flax rust. Biochem J. 1971 Jul;123(4):551–557. doi: 10.1042/bj1230551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Niyogi S. K. Biochemistry and physiology of bacterial ribonucleases. Prog Nucleic Acid Res Mol Biol. 1976;17:271–308. doi: 10.1016/s0079-6603(08)60073-2. [DOI] [PubMed] [Google Scholar]
- Harthoorn A. M., van der Walt K., Young E. Possible therapy for capture myopathy in captured wild animals. Nature. 1974 Feb 22;247(5442):577–577. doi: 10.1038/247577a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SHUSTER L., KHORANA H. G., HEPPEL L. A. The mode of action of ryegrass ribonuclease. Biochim Biophys Acta. 1959 Jun;33(2):452–461. doi: 10.1016/0006-3002(59)90135-0. [DOI] [PubMed] [Google Scholar]
- Scrubb L. A., Chakravorty A. K., Shaw M. Changes in the Ribonuclease Activity of Flax Cotyledons following Inoculation with Flax Rust. Plant Physiol. 1972 Jul;50(1):73–79. doi: 10.1104/pp.50.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. J., Maretzki A. Purification and properties of leaf ribonuclease from sugar cane. Biochim Biophys Acta. 1970 Aug 15;212(2):300–307. doi: 10.1016/0005-2744(70)90210-x. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Plant Nucleases. II. Properties of Corn Ribonucleases I and II and Corn Nuclease I. Plant Physiol. 1968 Sep;43(9):1339–1346. doi: 10.1104/pp.43.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Plant Nucleases: III. Polyacrylamide Gel Electrophoresis of Corn Ribonuclease Isoenzymes. Plant Physiol. 1971 Jul;48(1):64–68. doi: 10.1104/pp.48.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C. Polynucleotide-acrylamide gel electrophoresis of soluble nucleases from tobacco leaves. FEBS Lett. 1975 Mar 1;51(1):266–269. doi: 10.1016/0014-5793(75)80903-3. [DOI] [PubMed] [Google Scholar]