Abstract
Ethylene production was stimulated severalfold during the hypersensitive reaction of Samsun NN tobacco to tobacco mosaic virus (TMV). Exogenous methionine or S-adenosylmethionine (SAM) did not increase ethylene evolution from healthy or TMV-infected leaf discs, although both precursors were directly available for ethylene production. This indicates that ethylene production is not controlled at the level of methionine concentration or availability, nor at the level of SAM production or concentration. In contrast, 1-aminocyclopropane-1-carboxylic acid (ACC) stimulated ethylene production considerably. Thus, ethylene production is primarily limited at the level of ACC production.
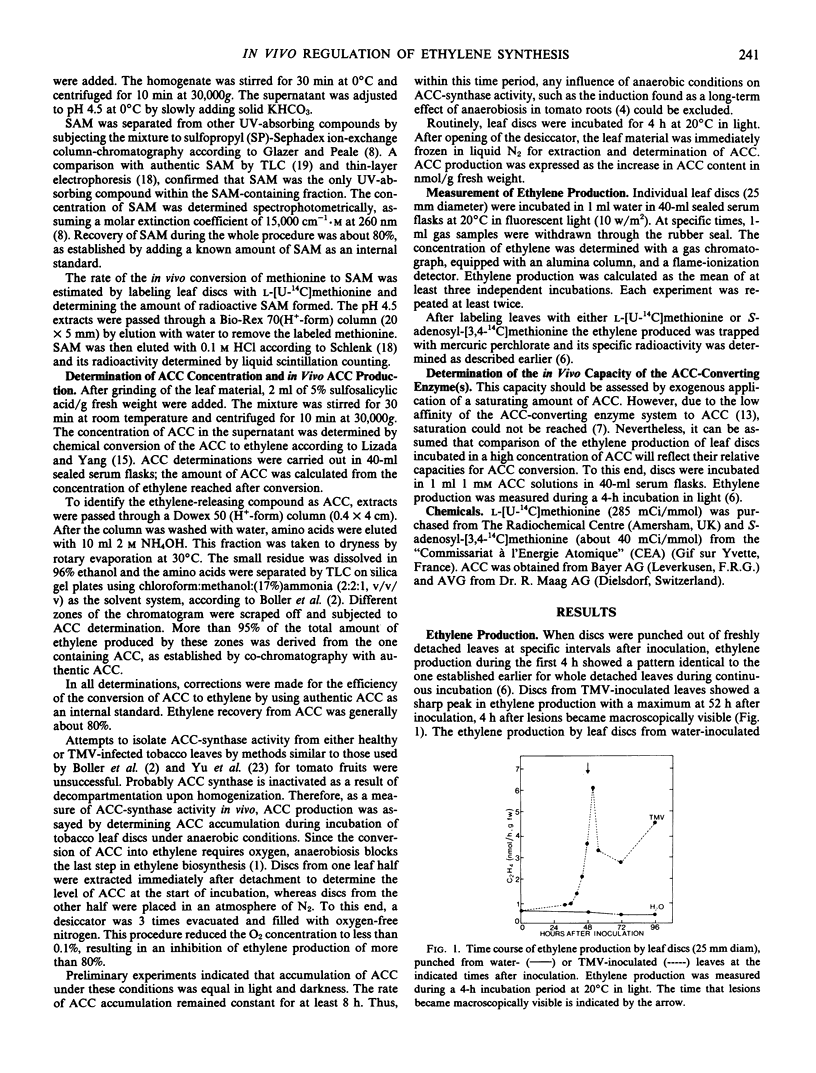
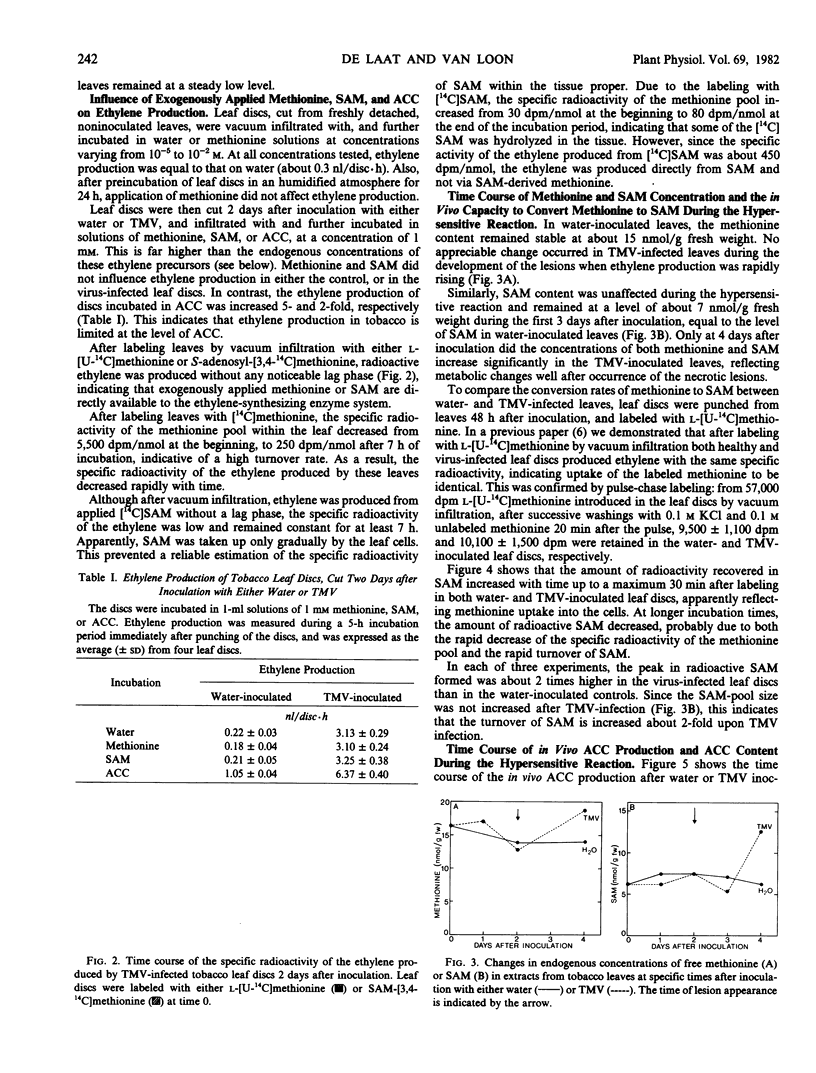
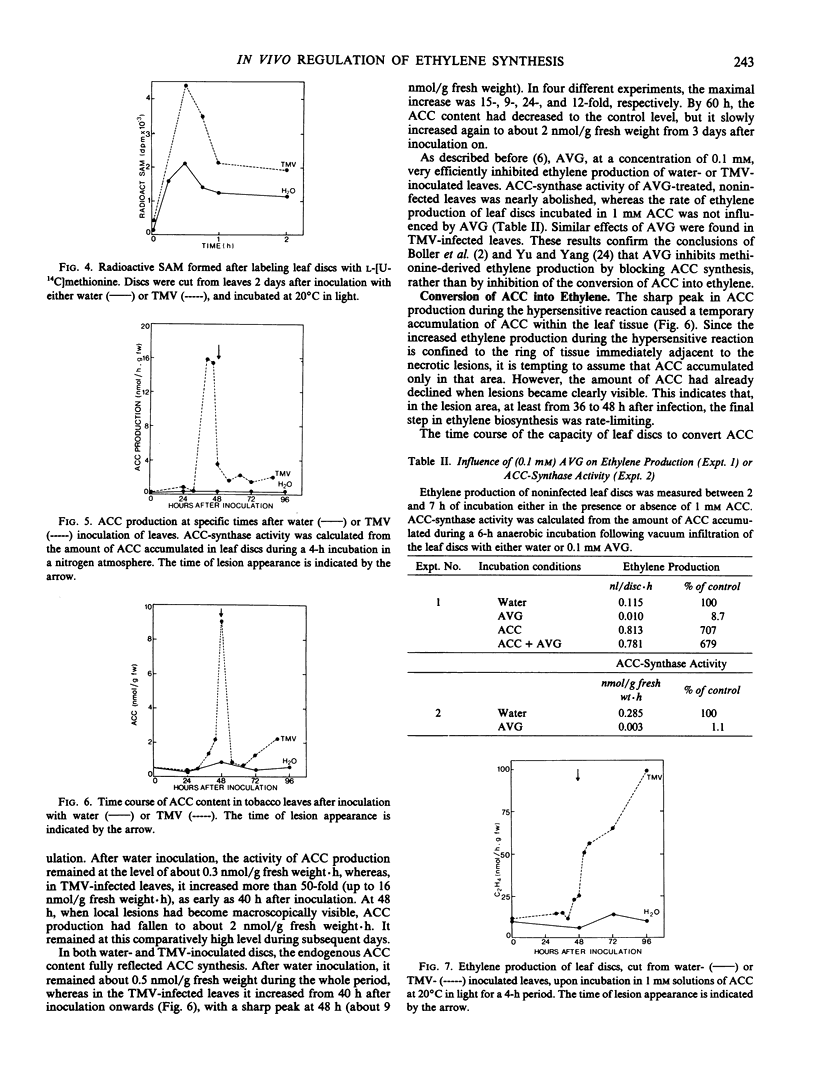
The regulation of ethylene production during the hypersensitive reaction to TMV was further studied by determining the time course of the concentrations of methionine, SAM, and ACC, as well as the course of their in vivo conversion rates. Endogenous concentrations of methionine and SAM remained unaffected until late in infection. On the contrary, the peak in ethylene production near the time of local lesion development was preceded by a large increase in ACC production. As a result of this increase, ACC accumulated in the leaf tissue. Only after local lesions became visible, the capacity to convert ACC into ethylene increased severalfold, associated with a sharp decrease in ACC content and a large increase in ethylene production.
Ethylene production in tobacco leaves reacting hypersensitively to TMV is thus regulated at the level of both the production of ACC and its conversion to ethylene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Yang S. F. Xylem Transport of 1-Aminocyclopropane-1-carboxylic Acid, an Ethylene Precursor, in Waterlogged Tomato Plants. Plant Physiol. 1980 Feb;65(2):322–326. doi: 10.1104/pp.65.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. Measurement of S-adenosyl-L-methionine levels by SP Sephadex chromatography. Anal Biochem. 1978 Dec;91(2):516–520. doi: 10.1016/0003-2697(78)90538-9. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Pritchard D. W., Ross A. F. The relationship of ethylene to formation of tobacco mosaic virus lesions in hypersensitive responding tobacco leaves with and without induced resistance. Virology. 1975 Apr;64(2):295–307. doi: 10.1016/0042-6822(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Schlenk F., Zydek C. R., Ehninger D. J., Dainko J. L. The production of S-adenosyl-L-methionine and S-adenosyl-L-ethionine by yeast. Enzymologia. 1965 Nov 6;29(3):283–298. [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Biosynthesis of wound ethylene. Plant Physiol. 1980 Aug;66(2):281–285. doi: 10.1104/pp.66.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat A. M., van Loon L. C. Regulation of Ethylene Biosynthesis in Virus-Infected Tobacco Leaves : I. DETERMINATION OF THE ROLE OF METHIONINE AS THE PRECURSOR OF ETHYLENE. Plant Physiol. 1981 Jul;68(1):256–260. doi: 10.1104/pp.68.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]




