Abstract
Tool use in extant primates may inform our understanding of the conditions that favoured the expansion of hominin technology and material culture. The ‘method of exclusion’ has, arguably, confirmed the presence of culture in wild animal populations by excluding ecological and genetic explanations for geographical variation in behaviour. However, this method neglects ecological influences on culture, which, ironically, may be critical for understanding technology and thus material culture. We review all the current evidence for the role of ecology in shaping material culture in three habitual tool-using non-human primates: chimpanzees, orangutans and capuchin monkeys. We show that environmental opportunity, rather than necessity, is the main driver. We argue that a better understanding of primate technology requires explicit investigation of the role of ecological conditions. We propose a model in which three sets of factors, namely environment, sociality and cognition, influence invention, transmission and retention of material culture.
Keywords: material culture, tool use, primates
1. Introduction
Tool use is widespread in the animal kingdom [1], but habitual tool use is restricted to only a few bird and mammal species, such as New Caledonian crows (Corvus moneduloides) and bottle-nosed dolphins (Tursiops sp.) [1]. Among non-human primates, frequent and diverse tool use is observed only in chimpanzees (Pan troglodytes) [2], orangutans (Pongo pygmaeus and Pongo abelii) [3], bearded capuchins (Sapajus libidinosus) [4] and, to a lesser extent, long-tailed macaques (Macaca fascicularis aurea) [5]. Given their close phylogenetic relatedness to humans, their tool use may provide insights into the conditions that favoured the extraordinary expansion of hominin technology.
Chimpanzees use a variety of tools in a range of contexts, including foraging, self-maintenance and social functions [2]. Orangutans use stick tools in similar contexts, especially on Sumatra [3]. Wild bearded capuchin monkeys living in savannah-like environments also use a variety of tools, including stones to crack open nuts and sticks to dig for tubers [4]. Lastly, island-dwelling long-tailed macaques use stones to crack molluscs, crabs and nuts [6].
The question is whether the use of tools in these primates can be termed ‘cultural’. It is important to resolve this question, because human technology is intrinsically cultural, as both the spread and maintenance of technological skills and knowledge are strongly dependent on social transmission [7].
The principal method used in wild animals to establish culture in nature has been the ‘method of exclusion’ [3,8]. This method identifies geographically variable behaviour patterns across long-term study sites and seeks to establish the presence of cultural variants by excluding behavioural variants that can be attributed to genetic or ecological differences across sites. This method has been used to demonstrate the presence of culture in wild populations, including chimpanzees and orangutans [3,8].
The main weakness of this method is that it cannot rule out ecology as an alternative explanation for behavioural variation [9]. Because unrecognized ecological differences may induce individuals to adopt different behavioural variants in the absence of any social transmission, this method has been applied as conservatively as possible by removing all variants with ecological correlates. However, as an unfortunate side effect, behavioural variants that have ecological (or genetic) correlates but are nonetheless culturally acquired will not be recognized as such. Importantly, social learning allows individuals to acquire behaviours appropriate to their ecological conditions, which implies that some important socially learned behaviours will be linked to the local environment.
In this opinion piece, we show how ecology affects primate material culture by influencing innovation, transmission and retention of tool-use behaviours in a population. We review findings on the influence of ecology on material culture in three habitual tool-using non-human primates: chimpanzees, orangutans and capuchin monkeys. By considering how not just social organization and cognitive capacities, but also the environment influence primate tool use [10], we may begin to disentangle the different determinants of material culture in non-human primates as well as humans. We conclude by presenting directions for future research and by providing a model describing the factors driving the evolution of primate material culture.
2. Ecology of culture matters
In line with the idea that necessity is the mother of invention, a link between fruit scarcity and an increase in certain types of tool use has been described for one chimpanzee population at Bossou, Guinea [11]. Similarly, a relationship between tool use and seasonal food scarcity has been suggested for bearded capuchins [12] and long-tailed macaques [6], although in neither case was an assessment of food availability carried out. Moreover, none of these studies tested alternative ecological hypotheses to explain tool-use patterns.
A number of recent studies [13–15] have explicitly addressed the role of ecological conditions in shaping primate foraging tool use by testing two main, not mutually exclusive, hypotheses [16]. The opportunity hypothesis states that encounter rates with tool materials and resources whose exploitation requires tools affect the likelihood of tool invention and frequency of tool use, thus explaining tool-use patterns. In contrast, the necessity hypothesis states that tool use is a response to scarcity of (preferred) foods [16,17]. As there is now overwhelming evidence from wild and captive studies that tool use in primates is socially learned [18], the following cases are assumed to be culturally transmitted.
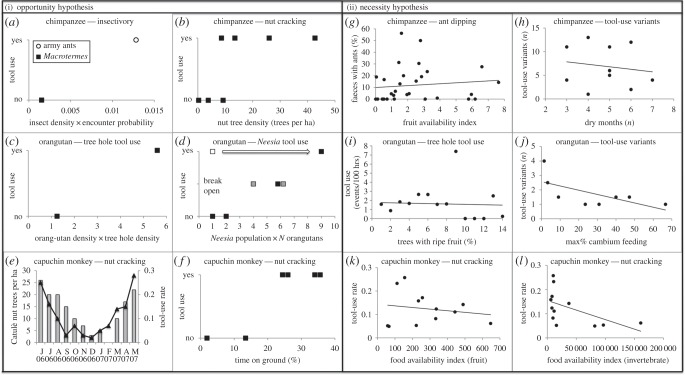
For chimpanzees, the opportunity hypothesis was supported at Seringbara, Guinea, where they use tools to harvest widely available army ants, but not to fish termites from rare and peripheral Macrotermes mounds [13]. Moreover, altitudinal overlap with ants, but not with termites, further increased opportunities to encounter ants (figure 1a and the electronic supplementary material, S1). In addition, at sites with higher (total) nut tree densities, chimpanzees were more likely to use tools to crack nuts (figure 1b and the electronic supplementary material, S1). The necessity hypothesis was not supported at Seringbara, as tool use in ant feeding did not increase at times of fruit scarcity (figure 1g and the electronic supplementary material, S1). Similarly, chimpanzees at Goualougo, Congo, did not compensate for seasonal lack of fruit by increasing tool use for harvesting social insects or honey [15]. Moreover, there was no correlation between the intensity of seasonality and the number of subsistence tool-use variants across chimpanzee study sites (figure 1h and the electronic supplementary material, S1).
Figure 1.
(i) Support for the opportunity hypothesis: (a) insectivory tool use by chimpanzees and opportunity for innovation (insect encounter likelihood) at Seringbara; (b) tool use in nut cracking and opportunity (nut tree densities) across chimpanzee sites; (c) tree hole tool use and opportunity (orangutan density × tree hole density) at two orangutan sites (Ketambe, Suaq); (d) Neesia tool use and opportunity (Neesia population size × N orangutans in Neesia population) across sites (black, Sumatran sites; grey, Bornean sites; white, Batang Toru, Sumatra); (e) tool-use rate to crack nuts by capuchin monkeys and opportunity (catulè nut availability) at Boa Vista (modified from reference [14]); (f) tool use in nut cracking and opportunity (% time on ground) across capuchin monkey sites. (ii) Lack of support for the necessity hypothesis: (g) tool use in ant dipping (% faeces with ants) in relation to fruit availability index by chimpanzees at Seringbara; (h) subsistence tool-use variants and number of dry months across chimpanzee sites; (i) tree-hole tool use in relation to % trees with ripe fruit by orangutans at Suaq; (j) subsistence tool-use variants and maximum % cambium feeding across orangutan sites; (k) tool-use rate to crack nuts in relation to food availability index (fruit, kg per ha) by capuchin monkeys at Boa Vista; (l) tool-use rate to crack nuts in relation to food availability index (invertebrate, kg per ha) by capuchin monkeys at Boa Vista.
In orangutans, intersite comparisons also supported the opportunity hypothesis [17]. First, tree-hole tool use was present at Suaq, but absent at Ketambe, consistent with 4.5 times more opportunities for such innovation at Suaq (figure 1c and the electronic supplementary material, S2). Second, orangutans were more likely to use tools to extract Neesia seeds at sites where opportunities for invention were higher (figure 1d and the electronic supplementary material, S2). The necessity hypothesis was not supported [17]. First, at Suaq, insect-extraction tool use was not negatively related to fruit availability (figure 1i and the electronic supplementary material, S2). Second, the number of subsistence tool-use variants across orangutan sites was not correlated with the incidence of extreme food scarcity, as indexed by maximum monthly percentage of time individuals feed on cambium (figure 1j and the electronic supplementary material, S2).
The opportunity hypothesis was also supported in bearded capuchin monkeys at Boa Vista, Brazil [14]. Monthly tool-use rate was correlated with the availability of the most exploited species of palm nuts (catulè nuts, Attalea barreirensis; figure 1e and the electronic supplementary material, S3). Moreover, stone tool use was related to degree of terrestriality (i.e. opportunities to encounter nuts and stones), because it has been reported only at sites where individuals spend a considerable amount of time on the ground (figure 1f and the electronic supplementary material, S3; modified from reference [19]). In contrast, the necessity hypothesis was not supported. Tool-use rate was not correlated with availability of fruits and invertebrates (figure 1k,l and the electronic supplementary material, S3).
The conclusion from these studies regarding the ecological influences on feeding tool use in three different taxa is that opportunity, not necessity, is the main driver. We showed that (ecological) opportunities influence occurrence of tool use, and likely the species' cultural repertoires. The resources extracted using tools (nuts, honey, insects) are among the nutritionally richest in primate habitats. Hence, extraction pays off, and not just during times of food scarcity.
3. Future directions
The above-mentioned results do not support the necessity hypothesis. Instead, the results support the opportunity hypothesis: the more exposure a population has to opportunities to invent and practice novel tool use, the more likely the behaviour occurs. We reviewed all the available studies testing the two hypotheses in non-human primates. However, because the number of studies is small, additional testing is needed.
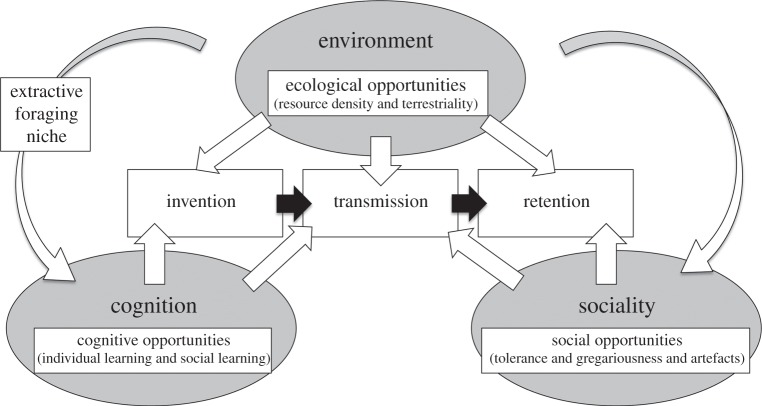
We use these findings to propose a model in which three sets of factors, namely environment, sociality and cognition, influence invention, transmission and retention of material culture (figure 2). First, the environment provides ecological opportunities, in terms of resource density and likelihood to encounter them, which prompt innovation, transmission and retention of tool use. Second, social opportunities for tool use in terms of social tolerance, gregariousness and leftover artefacts from tool-use activities [20] influence transmission and retention of tool use. Third, cognitive capacities for tool use in terms of individual and social learning abilities are also important. In innovation, individual learning plays a crucial role, whereas socially biased learning is essential for transmission of tool use among group members.
Figure 2.
The three-factor model of primate material culture (modified from reference [10] by adding ‘environment’ and ‘retention’). White arrows, direct influence; black arrows, causal sequence.
The proposed model provides a framework for future research on the emergence and distribution of material culture across primate, as well as non-primate species [21]. It provides a unifying perspective on the emergence of tool use, which may help to explain variation in tool-use diversity and complexity across populations. Lastly, the model allows us to further assess the roles of the environment, sociality and cognition across species with varying social systems and ecological settings.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
K.K. thanks Lucie Burgers Foundation (The Netherlands), Homerton College (Cambridge) and Tetsuro Matsuzawa. C.P.v.S. thanks the A.H. Schultz Foundation and SNF (grant no. 31003A-138368/1). Maria van Noordwijk, Serge Wich and Perry van Duijnhoven contributed unpublished information. E.V. thanks Noemi Spagnoletti and Michele Verderane for raw data on which some figures are based. Lastly, we thank two anonymous reviewers for helpful comments on an earlier version of the manuscript.
References
- 1.Shumaker RW, Walkup KR, Beck BB. 2011. Animal tool behavior: the use and manufacture of tools by animals. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 2.McGrew WC. 2004. The cultured chimpanzee: reflections on cultural primatology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105. ( 10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 4.Ottoni EB, Izar P. 2008. Capuchin monkey tool use: overview and implications. Evol. Anthropol. 17, 171–178. ( 10.1002/evan.20185) [DOI] [Google Scholar]
- 5.Malaivijitnond S, Lekprayoon C, Tandavanittj N, Panha S, Cheewatham C, Hamada Y. 2007. Stone-tool usage by Thai long-tailed macaques (Macaca fascicularis). Am. J. Primatol. 69, 227–233. ( 10.1002/ajp.20342) [DOI] [PubMed] [Google Scholar]
- 6.Gumert MD, Kluck M, Malaivijitnond S. 2009. The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea region of Thailand. Am. J. Primatol. 71, 594–608. ( 10.1002/ajp.20694) [DOI] [PubMed] [Google Scholar]
- 7.Tomasello M. 1999. The cultural origins of human cognition. Cambridge, MA: Harvard University Press. [Google Scholar]
- 8.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 2001. Charting cultural variation in chimpanzees. Behaviour 138, 1481–1516. ( 10.1163/156853901317367717) [DOI] [Google Scholar]
- 9.Laland KN, Janik VM. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547. ( 10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 10.van Schaik CP, Deaner RO, Merrill M. 1999. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741. ( 10.1006/jhev.1999.0304) [DOI] [PubMed] [Google Scholar]
- 11.Yamakoshi G. 1998. Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: possible implications for ecological importance of tool-use. Am. J. Phys. Anthropol. 106, 283–295. () [DOI] [PubMed] [Google Scholar]
- 12.Moura ACA, Lee PC. 2004. Capuchin stone tool use in Caatinga dry forest. Science 306, 1909 ( 10.1126/science.1102558) [DOI] [PubMed] [Google Scholar]
- 13.Koops K, McGrew WC, Matsuzawa T. 2013. Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees (Pan troglodytes verus)? Anim. Behav. 85, 175–185. ( 10.1016/j.anbehav.2012.10.022) [DOI] [Google Scholar]
- 14.Spagnoletti N, Visalberghi E, Verderane MP, Ottoni E, Izar P, Fragaszy D. 2012. Stone tool use in wild bearded capuchin monkeys, Cebus libidinosus. Is it a strategy to overcome food scarcity? Anim. Behav. 83, 1285–1294. ( 10.1016/j.anbehav.2012.03.002) [DOI] [Google Scholar]
- 15.Sanz CM, Morgan DB. 2013. Ecological and social correlates of chimpanzee tool use. Phil. Trans. R. Soc. B 368, 20120416 ( 10.1098/rstb.2012.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox EA, Sitompul AF, van Schaik CP. 1999. Intelligent tool use in Sumatran orangutans. In The mentalities of gorillas and orangutans (eds Parker ST, Mitchell RW, Miles HL.), pp. 99–116. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Fox EA, van Schaik CP, Sitompul AF, Wright DN. 2004. Intra- and interpopulational differences in orangutan (Pongo pygmaeus) activity and diet: implications for the invention of tool use. Am. J. Phys. Anthropol. 125, 162–174. ( 10.1002/ajpa.10386) [DOI] [PubMed] [Google Scholar]
- 18.Whiten A. 2012. Social learning, traditions, and culture. In The evolution of primate societies (eds Mitani J, Call J, Kappeler PM, Palombit RA, Silk JB.), pp. 682–700. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 19.Spagnoletti N. 2009. Uso di strumenti in una popolazione di Cebus libidinosus allo stato selvatico in Piauí, Brasile. Rome, Italy: Università La Sapienza di Rome. [Google Scholar]
- 20.Fragaszy D, Biro D, Eshchar Y, Humle T, Izar P, Resende B, Visalberghi E. 2013. The fourth dimension of tool use: temporally enduring artefacts aid primates learning to use tools. Phil. Trans. R. Soc. B 368, 20120410 ( 10.1098/rstb.2012.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sargeant BL, Wirsing AJ, Heithaus MR, Mann J. 2007. Can environmental heterogeneity explain individual foraging variation in wild bottlenose dolphins (Tursiops sp.)? Behav. Ecol. Sociobiol. 61, 679–688. ( 10.1007/s00265-006-0296-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.