Abstract
Climate warming is predicted to cause many changes in ectotherm communities, one of which is phenological mismatch, wherein one species' development advances relative to an associated species or community. Phenological mismatches already lead to loss of pollination services, and we predict that they also cause loss of biological control. Here, we provide evidence that a pest develops earlier due to urban warming but that phenology of its parasitoid community does not similarly advance. This mismatch is associated with greater egg production that likely leads to more pests on trees.
Keywords: climate change, urban ecology, ecosystem services, parasitoid
1. Introduction
Climate warming can cause phenological mismatches between associated species. These mismatches can disrupt trophic interactions and reduce ecosystem services [1]. For example, when flowers open before bees become active, pollination is reduced, and plants produce fewer seeds [2]. Warming could also reduce biological control of pests if pest phenology advances more than predator or parasitoid phenology. If mismatches between predators and prey occur anywhere, it is probably in cities where air temperatures are up to 12°C hotter than in surrounding areas [3]. Empirically, herbivorous insect pests are often more abundant on urban than rural plants. In part, this is because warming increases their survival, fecundity and population growth rate relative to individuals in cooler urban areas [4,5]. However, high pest abundance in cities could also result from poor biological control [6] due to phenological mismatches between pests and natural enemies.
In a previous study, we found that the scale insect Parthenolecanium quercifex (Hemiptera: Coccidae) is 12 times more abundant on trees in the warmest urban habitats [4]. Female P. quercifex are attacked by several hymenopteran parasitoids that kill them or reduce their fecundity. In this study, we monitored P. quercifex and parasitoid phenology in hot urban sites and in similar urban sites that were cooler, relative to the hot sites. We tested if warming reduced parasitism rate or changed parasitoid reduction of P. quercifex egg number.
2. Material and methods
(a). Study organisms
Parthenolecanium quercifex is a common, native, univoltine pest on urban oak trees. In North Carolina, USA, adults produce eggs for about three weeks between April and May on willow oak (Quercus phellos). Females die after depositing eggs underneath their body cavities. Eggs hatch before June.
Parasitoids develop in P. quercifex throughout its life cycle [7]. Our study focused on species that are within P. quercifex during oviposition in April and May. Although the specifics of the parasitoids' life cycles are not known, adults are absent in early spring and become more abundant throughout the season, which suggests parasitoids overwinter in P. quercifex as larvae. To confirm identities of P. quercifex parasitoids in our study area, Raleigh, NC, USA, we collected 20 P. quercifex from each of 16 trees across the city on 16 April 2012 and placed them in individual 1.5 ml vials with cotton plugs, in an incubator (25°C and 70% RH) until eclosion. We identified parasitoids to genus and deposited voucher specimens at the North Carolina State University Insect Museum.
(b). Study system
Previously, we studied P. quercifex abundance on willow oaks at 40 sites in Raleigh [4]. We overlaid a surface temperature layer with a tree inventory in ArcGIS (ESRI 2011) to choose study sites. We selected 20 sites from the hottest zones on the map and 20 sites from the coolest zones with two adjacent willow oak trees. For this study, we chose eight hot and eight cold sites that had detectable numbers of P. quercifex from our original 40 sites. One of our hot sites was removed from the study due to construction that interrupted data collection. Although other variables, such as pollution, water availability, and pesticide and fertilizer runoff could have contributed to host and parasitoid abundance and phenology, we assumed that temperature was the dominant effect. As discussed below, including site as a random effect in our models at least partially accounted for these variables. We placed an ibutton thermachron (model DS1921G, precision: 0.5°C, accuracy:±1.0°C; Dallas Semiconductor, TX, USA) on an outer branch of one tree at each site from February to May 2014 to confirm that air temperatures at hot sites were higher than those at cold sites. The ibuttons recorded temperature every hour. Ibuttons were lost at five sites and malfunctioned at three sites. We present temperature data from five cold and three hot sites. To compare average temperatures at hot and cold sites, we used a linear mixed effects model in the nlme package R [8] with temperature category and date as factors and site as a random effect. We compared autoregressive, unstructured and compound symmetry correlation structures, and chose the autoregressive structure based on Akaike's Information Criterion (AIC). Hereafter, models of this type were considered significantly different when ΔAIC was more than 2. Then we compared models with all predictor combinations, including interaction terms, using AICc. We used this method for all linear mixed effects model selections.
(i). Prediction 1: Parthenolecanium quercifex develops earlier in warmer urban areas but parasitoids do not
On 2 and 18 March, 1 and 16 April and 1 May, we collected between 10 and 20 P. quercifex from two adjacent trees at each of our 16 study sites. We categorized each P. quercifex as second instar, adult, adult with eggs, or dead adult with eggs and dissected each to look for parasitoid larvae. We tested our prediction with a generalized linear mixed model in the GLIMMIX Procedure of SAS (SAS Institute 2013). Date, categorical temperature and their interaction were fixed effects, and ‘site’ was a random effect. To determine how temperature effects per cent parasitism and parasitoid larval phenology, we used logit-transformed (log((x + 1)/(1 − x + 1)) proportion of scales with parasitoid larvae as a response, temperature and date as predictors, and site as a random effect in a linear mixed effects model.
We placed one 7.6 × 12.7 cm yellow sticky card (Olson Products, OH, USA) in both trees at each site, each time we collected P. quercifex life stage data to determine parasitoid flight phenology at hot and cold sites. We collected cards on the next date and counted and identified P. quercifex parasitoids. We used log(x + 1)-transformed parasitoid count on sticky cards as a response, temperature and date as predictors, and site as a random effect in a linear mixed effects model to determine how temperature affected parasitoid abundance and parasitoid flight phenology.
(ii). Prediction 2: Parasitoids reduce Parthenolecanium quercifex egg count more in cold than in hot urban areas
We harvested P. quercifex egg sacs from sites used in Prediction 1 along with three additional hot sites and two additional cold sites from our original study [4] to add replicates, for a total of 10 hot and 10 cold sites. At each site, we collected 40 dead females on 23–24 April 2012, before P. quercifex eggs hatched. Females and their eggs were inspected for live parasitoids and parasitoid exit holes and were placed into vials with 95% ethanol. We put eggs from each sample into a Petri dish with 10 ml of 95% ethanol and photographed them using a Canon EOS 7D camera with a Canon EF-S compact 50 mm macro lens. We used ImageJ to count area occupied by eggs in each photo and calculated the area of 10 eggs in five random Petri dishes from each site to get average egg size per site. We divided egg area per dish by average egg size to calculate egg count.
Different parasitoid communities attack P. quercifex during each life stage. We focused on parasitoid species that are within P. quercifex during oviposition. We separated the dataset into two categories: P. quercifex with eggs and P. quercifex without eggs. With those that produced eggs, we tested for the effects of warming and parasitoid presence on egg count with a generalized linear mixed model in the GLIMMIX Procedure of SAS. Parasitoids and temperature were fixed effects, and site was a random effect. We assumed that the data were drawn from a gamma distribution, and the log of the expected mean response was a function of the fixed effects.
3. Results
A discussion of parasitoid identities and communities is in the electronic supplementary material, figure S1a,b.
(a). Temperature
Sites designated as ‘hot’ were significantly warmer than ‘cold’ sites (mean ± s.e.m. 19.0 ± 0.5°C and 18.5 ± 0.5°C, respectively; F1,8 = 5.51, p = 0.0469; electronic supplementary material, figure S2a,b), depending on day (ΔAICc = 296.72; assigned temp. × date: F103,748 = 2.44, p < 0.0001) wherein the hot and cold sites were more different earlier in the year than later.
(i). Prediction 1: Parthenolecanium quercifex develops earlier in warmer urban areas but parasitoids do not
The effects of temperature on the proportion of P. quercifex producing eggs depended on date (GLIMMIX model; F3,45 = 3.91, p = 0.0146), such that a higher proportion of P. quercifex at hot sites produced eggs on early dates (figure 1a). This indicates that P. quercifex produces eggs earlier at hot sites, supporting our prediction that warming advances P. quercifex phenology.
Figure 1.

(a) Parthenolecanium quercifex produces eggs early in warmer parts of the city, (b) but emergence timing of adult parasitoids is not affected. (Hot sites, pink; cold sites, blue.)
Date was the only significant factor predicting the proportion of P. quercifex with parasitoid larvae (figure 2a; ΔAICc = 2.36; F1,42 = 98.52, p < 0.0001), indicating that parasitoid larvae did not appear earlier at hot sites and that percent parasitism was not different between hot and cold sites. As might be expected given the higher density of P. quercifex at hot sites, adult parasitoid abundance was also higher at hot sites (figure 1b; F1,13 = 9.24, p < 0.0095) and significantly increased as the season progressed (F1,53 = 174.55, p < 0.0001), but the best model did not include an interaction between temperature and time (ΔAICc = 3.35). Collectively, our data show that parasitoid abundance increased with P. quercifex abundance, but that percent parasitism and parasitoid phenology were not significantly different at hot and cold sites.
Figure 2.
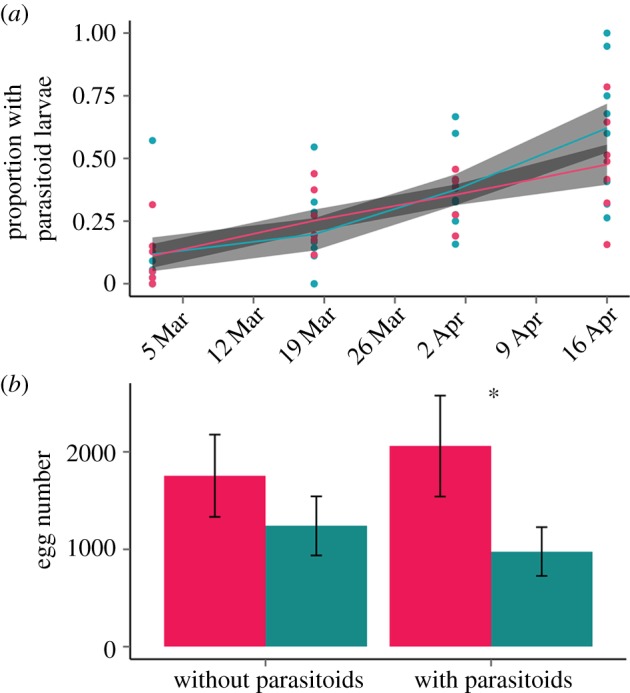
(a) The same proportion of P. quercifex are parasitized across urban temperatures, but (b) Parthenolecanium quercifex in hot urban zones produce twice as many eggs when parasitized (hot sites, pink; cold sites, blue; asterisk indicates significant difference within the parasitoid treatment).
(ii). Prediction 2: Parasitoids reduce Parthenolecanium quercifex egg count more in cold than in hot urban areas
Temperature and parasitism interacted to affect egg count (figure 2b; F1,589 = 3.73, p = 0.0258), as parasitized P. quercifex that oviposited produced twice as many eggs at hot sites than at cold sites. Overall, egg numbers were not different between hot and cold sites (F1,17 = 1.96, p = 0.1799).
4. Discussion
Ecologists have predicted for several decades that insect pests will become more abundant as the earth warms [9]. We show for the first time that pests and their parasitoids in the city undergo phenological mismatches akin to those predicted to occur due to global warming [10]. The scale insect P. quercifex oviposited earlier in warmer urban areas, relative to similar, cooler areas, whereas the phenology of its parasitoid community did not similarly advance. This mismatch between parasitoid and host phenology did not reduce the proportion of P. quercifex scale insects that were parasitized. However, parasitized P. quercifex on trees in hot zones produced twice as many eggs as parasitized individuals on cooler urban trees, while the number of eggs produced by unparasitized individuals did not differ with temperature, as found in previous studies of other scale insect species [5]. Overall, we provide evidence that phenological mismatches between scale insects and their parasitoids could lead to more pests on street trees.
At least two factors may contribute to the phenological mismatches that we observe. First, P. quercifex are mostly sedentary, and so have no means to escape or moderate high temperatures. By contrast, most natural enemies can move at greater spatial scales and through hot and cold zones within the city. Even at small spatial scales, mobile natural enemies like parasitoids can buffer high temperatures by moving to cooler parts of their habitats [11]. In our system, this may mean moving deeper into the canopy or within bark crevices. Additionally, endoparasitoids may be buffered from extreme temperatures, because they develop within their hosts [12].
This study documents a new mechanism for urban pest outbreaks, which damage trees and reduce ecosystem services [13]. Warming may increase pest abundance in many habitats through multiple mechanisms, including phenological mismatches that reduce biological control.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Andrew Ernst and George Washburn collected data.
Disclaimer
EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA.
Data accessibility
Data will be deposited in the NCEAS repository.
Funding statement
This study was supported by the Department of The Interior Southeast Climate Science Center under Cooperative Agreement Numbers G11AC20471 and G13AC00405, grant 2013-02476 from USDA AFRI to S.D.F., and the NCSU Department of Entomology. R.R.D. thanks NSF0953390 and NSF1136703. The research described in this paper has been funded wholly or in part by the United State Environmental Protection Agency under the Science to Achieve Results Graduate Fellowship Program.
References
- 1.Kudo G, Ida TY. 2013. Early onset of spring increases the phenological mismatch between plants and pollinators . Ecology 94, 2311–2320. ( 10.1890/12-2003.1) [DOI] [PubMed] [Google Scholar]
- 2.Thomson JD. 2010. Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte . Phil. Trans. R. Soc. B 365, 3187–3199. ( 10.1098/rstb.2010.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oke T. 1973. City size and the urban heat island . Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 4.Meineke EK, Dunn RR, Sexton JO, Frank SD. 2013. Urban warming drives insect pest abundance on street trees . PLoS ONE 8, e59687 ( 10.1371/journal.pone.0059687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dale AG, Frank SD. 2014. Urban warming trumps natural enemy regulation of herbivorous pests. Ecol. Appl. 24, 1596–1607. ( 10.1890/13-1961.1) [DOI] [PubMed] [Google Scholar]
- 6.Hanks L, Denno R. 1993. Natural enemies and plant water relations influence the distribution of an armored scale insect . Ecology 74, 1081–1091. ( 10.2307/1940478) [DOI] [Google Scholar]
- 7.Schultz PB. 1984. Natural enemies of oak lecanium (homoptera: Coccidae) in eastern Virginia . Environ. Entomol. 13, 1515–1518. [Google Scholar]
- 8.R Development Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org. [Google Scholar]
- 9.Porter J, Parry M, Carter T. 1991. The potential effects of climatic change on agricultural insect pests . Agric. Meteorol. 57, 221–240. ( 10.1016/0168-1923(91)90088-8) [DOI] [Google Scholar]
- 10.Hance T, van Baaren J, Vernon P, Boivin G. 2007. Impact of extreme temperatures on parasitoids in a climate change perspective . Annu. Rev. Entomol. 52, 107–126. ( 10.1146/annurev.ento.52.110405.091333) [DOI] [PubMed] [Google Scholar]
- 11.Schultz T. 1998. The utilization of patchy thermal microhabitats by the ectothermic insect predator, Cicindela sexguttata . Ecol. Entomol. 23, 444–450. ( 10.1046/j.1365-2311.1998.00154.x) [DOI] [Google Scholar]
- 12.Péré C, Jactel H, Kenis M. 2013. Response of insect parasitism to elevation depends on host and parasitoid life-history strategies. Biol. Lett. 9, 20130028 ( 10.1098/rsbl.2013.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dale AG, Frank SD. 2014. The effects of urban warming on herbivore abundance and street tree condition. PLoS ONE 9, e102996 ( 10.1371/journal.pone.0102996) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be deposited in the NCEAS repository.


