Abstract
Sperm senescence can have important evolutionary implications due to its deleterious effects on sperm quality and offspring performance. Consequently, it has been argued that polyandry (female multiple mating) may facilitate the selection of younger, and therefore competitively superior, sperm when ejaculates from multiple males compete for fertilization. Surprisingly, however, unequivocal evidence that sperm ageing influences traits that underlie sperm competitiveness is lacking. Here, we used a paired experimental design that compares sperm quality between ‘old’ and ‘young’ ejaculates from individual male guppies (Poecilia reticulata). We show that older sperm exhibit significant reductions in sperm velocity compared with younger sperm from the same males. We found no evidence that the brightness of the male's orange (carotenoid) spots, which are thought to signal resistance to oxidative stress (and thus age-related declines in sperm fitness), signals a male's ability to withstand the deleterious effects of sperm ageing. Instead, polyandry may be a more effective strategy for females to minimize the likelihood of being fertilized by aged sperm.
Keywords: sperm age, sperm viability, sperm velocity, sperm competition, postcopulatory sexual selection
1. Introduction
Ageing can have multiple deleterious effects on fitness, extending from the whole organism to cellular-level processes, including gamete function. Post-meiotic sperm senescence is the gradual accumulation of damage during the lifespan of sperm and is caused by a number of factors, most notably oxidative stress attributable to an imbalance in the accumulation of reactive oxygen species. Sperm cells are particularly vulnerable to oxidative damage because they are rich in mitochondria and polyunsaturated fatty acids, and possess a small cytosol with relatively limited capacity for self repair [1–3].
Recent interest from evolutionary biologists in post-meiotic sperm senescence centres around the deleterious effects of sperm ageing on a range of fitness traits, including fertilization ability [4] and offspring performance [5,6]. In particular, the potential for sexual selection to act on female traits that mitigate the costs of mating with males with impaired (aged) sperm has been explored from a theoretical perspective [2,7]. This work suggests that females may benefit either by basing their mating preferences on traits that reveal information about sperm ageing-related quality (precopulatory selection), or by mating multiply to maximize the likelihood that aged (competitively inferior) sperm are unlikely to fertilize their eggs (postcopulatory selection). Both scenarios generate the prediction that sperm senescence is related to sperm quality, but the precopulatory selection hypothesis generates the additional requirement that males advertise their ability to protect sperm from senescence though the expression of sexually selected traits.
In this paper, we test the twofold predictions (i) that sperm ageing has deleterious effects on sperm quality and (ii) that male sexual ornamentation reflects an individual's ability to mitigate the effects of sperm ageing on sperm quality. We use guppies (Poecilia reticulata), a highly polyandrous livebearing fish, to test both predictions within a paired experimental framework that manipulates sperm age during male sperm storage without confounding sperm age with mating history or male age. To address the first aim, we experimentally manipulated sperm age in vivo through repeated sperm (or sham) extractions in order to test how sperm age influences sperm viability (the proportion of live relative to dead sperm in the ejaculate) and sperm swimming velocity (a proxy for sperm competitiveness in guppies [8] and other species [9,10]). To address our second aim, we determined whether carotenoid-based colour pigments reflect a male's ability to protect sperm quality (viability and velocity) from the deleterious consequences of sperm ageing, and thus whether male secondary sexual traits can reveal information about sperm age-related quality during mate choice. Carotenoid-based colour spots are used by female guppies to assess male attractiveness [11] and are thought to signal resistance to oxidative stress in sperm [12]. Accordingly, males with bright carotenoid-based coloration should be better equipped to protect their sperm from oxidative stress, and hence from the deleterious effect of sperm ageing, than their drab counterparts.
2. Material and methods
(a). Study species and its maintenance
The guppies used in this experiment were descendants of fish captured from Alligator Creek River in Queensland, Australia. Stocks were maintained in mixed-sex groups (approx. 1 : 1 sex ratio) at 26 ± 1°C and fed a mix of Artemia nauplii and commercial flake food.
(b). Experimental design
Six-month-old males (n = 32) were individually isolated in 1 l plastic tanks prior to the experiment in order to standardize recent mating history and social environment. After 7 days, all males were stripped to empty their sperm reserves (hereafter ‘strip 0’, see figure 1).
Figure 1.
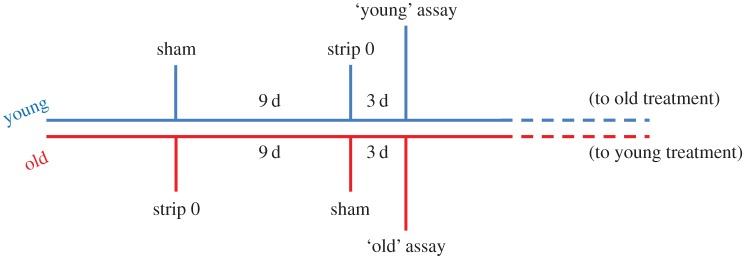
Schematic representation of the experimental design. (Online version in colour.)
The experiment consisted of a paired design in which sperm quality traits from each male were assessed twice under two conditions: ‘young’, where sperm were collected 3 days after the initial strip (strip 0), and ‘old’, where sperm were collected 12 days post-stripping. To balance the order in which young and old sperm were tested, half of the males were tested in the young treatment first, while the other half experienced the old treatment first. Once the first test was concluded the males were rested and housed for 1 week with non-virgin females (which are generally sexually unresponsive but would have maintained the males' sexual interest). The females were rotated among males so that any difference in the females' behaviour or attractiveness to the males was minimized. Finally, to standardize the number of times males underwent the stripping procedure (including anaesthesia), a ‘sham’ stripping procedure was performed on day 9 (i.e. the same time as males in the ‘young’ treatment) for males in the old treatment (see figure 1). The sham procedure was similar to the real stripping procedure, except that pressure was applied on a slightly different part of the male's abdomen that did not result in sperm discharge.
(c). Sperm traits assays
We collected ejaculates and assessed sperm quality using standard procedures [13]. Sperm curvilinear velocity (VCL, μm s−1), which predicts competitive fertilization success in guppies [8], was measured using a CEROS sperm tracker (Hamilton-Thorne Research, Beverly, MA, USA). Sperm viability, the proportion of live and dead sperm in the ejaculate, was assessed using a live/dead viability kit (L-7011, Molecular Probes Inc., Eugene, OR, USA) on a minimum of 100 sperm cells from each male.
(d). Colour pattern analyses
The left side of each male was photographed using a digital SLR camera (Nikon D70s) fitted with a 105 mm macro lens (AF-S f/2.8 VR Micro-Nikkor). We used the same camera settings throughout (aperture: f/13, ISO: 200, shutter speed: 1/4 s.). Illumination was provided by two bench top fluorescence lamps (each containing 2 × 9 W Osram Dulux bulbs), angled at 45° to reduce specular reflectance. A simulated Gretag Macbeth colour standard with known reflectance properties was included in each image to provide a reference for the imaging software. Each image was captured in RAW format (.NEF file) and subsequently converted to TIFF format for import into the software package ColourWorker (http://www.chrometrics.com). ColourWorker was used to measure the reflectance spectra of the male's carotenoid spots (orange/yellow coloration) by selecting guppy-specific orange spectra as references (for details see [14]). We took three measures for each spot, which were used to calculate the mean reflectance for each fish. Spot brightness was then measured by calculating the area under the reflectance spectrum (i.e. integrating over the wavelength range 400–700 nm). We also tested the effectiveness of the ColourWorker program in evaluating the brightness of orange coloration by comparing the spectral output of the program with reflectance data obtained using a spectrometer (see the electronic supplementary material).
(e). Statistical analysis
We used paired t-tests to test for differences in sperm motility between sperm of different ages (‘young’ and ‘old’) obtained from the same male. These tests were run separately for sperm velocity and sperm viability (arcsine square root transformed). A linear mixed-effects model (LMM) was used to test whether the brightness of a male's orange coloration can reflect his ability to mitigate the effects of ageing on sperm quality. In the model, treatment (young or old) was entered as fixed factor and the brightness of the orange spots was entered as a covariate. Male identity was included as random effect to account for the non-independence of data collected from the same male. Data were analysed using the statistical software R v. 3.0.3 (http://www.r-project.org).
3. Results
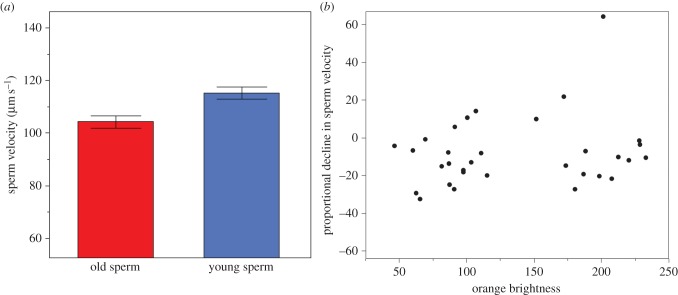
Within the same male, young sperm swam faster than old sperm (mean µm s−1 ± s.e., young sperm: 115.33 ± 2.31, old sperm: 104.35 ± 2.35, paired t-test: t31 = 3.33, p = 0.001, figure 2). However, we found no differences in sperm viability (proportion mean ± s.e., young sperm: 0.71 ± 0.03, old sperm: 0.70 ± 0.04, paired t-test: t28 = 0.040, p = 0.968) between treatments. The order in which sperm were tested (old or young first) had no effect on either of the sperm traits (both p > 0.27).
Figure 2.
(a) Mean (±s.e.) of sperm velocity (VCL, µm s−1) of young (3 days) and old (12 days) sperm. (b) Relationship between brightness of orange coloration and decline in sperm velocity due to sperm age (negative values indicate a decline in sperm velocity from young to old sperm). (Online version in colour.)
We found no effect of orange brightness on the relationship between sperm age and sperm swimming velocity (LMM: treatment: t = 2.344, p = 0.026, brightness: t = 0.855, p = 0.396, treatment × brightness: t = −1.454, p = 0.157) or sperm viability (treatment: t = 1.281, p = 0.211, brightness: t = 0.972, p = 0.335, treatment × brightness: t = −1.370 p = 0.182).
4. Discussion
Our findings reveal that as sperm age inside the male (during male sperm storage), sperm quality becomes impaired. By comparing old and young ejaculates within the same male, and hence accounting for inter-male variability in sperm traits [13,15], we found that old sperm exhibited impaired sperm velocity compared with young sperm. As we did not use natural matings to manipulate sperm ageing, we were able to disentangle the effects of sperm ageing per se from variance attributable to mating history and male–female social interactions. As sperm velocity is a determinant of competitive fertilization success in guppies [8], our findings suggest that males with aged sperm are likely to be less successful in sperm competition than those that are able to provide fresh sperm [8]. In guppies, where sperm competition is intense [16], any decline in sperm competitiveness is likely to represent a significant reduction in a male's reproductive fitness, and may fuel the evolution of male strategies to limit such costs (e.g. elevated mating rates). Differences in sperm velocity due to sperm ageing may also explain the high variability among ejaculates from the same male often found in experiments [17].
We also investigated whether the link between sperm ageing and carotenoid coloration may help explain the female's preference for colourful males. Specifically, we tested for a relationship between the brightness of a male's orange spots (which are rich in antioxidants) and the ability to protect sperm from the deleterious effects of ageing (thought to be caused by oxidative stress; [12]). Evidence supporting such a scenario comes from two studies of birds [18] and fish [19] where female choice plays a major role in the mating system. In guppies, males typically circumvent female choice by using forced copulations, and therefore females may have limited capacity to rely on precopulatory mate choice to discriminate among males. However, we acknowledge that our findings for this portion of the study are correlational and demand experimental approaches (e.g. manipulating the amount of antioxidants available to males) to discount the precopulatory mate choice hypothesis. Furthermore, it is possible that female guppies use other mechanisms to select for males with young sperm at the precopulatory level, for example, by choosing successful males that have a high turnover of sperm and are therefore more likely to deliver ejaculates containing fresh sperm [20].
In conclusion, we found that male sperm storage has a deleterious effect on sperm velocity, which is likely to have important fitness implications for male guppies [8]. Furthermore, we find no evidence that orange brightness signals a male's ability to protect his sperm from the deleterious effect of ageing. This latter finding suggests that any potential fitness benefits of mating with males with young sperm are unlikely to be generated by precopulatory female choice. Instead, by mating multiply, females may exploit postcopulatory mechanisms to favour young sperm in the competition to fertilize eggs.
Supplementary Material
Acknowledgements
We thank Alessandro Devigili for providing the guppy colour spot reference spectra. We are also grateful to two anonymous referees for their helpful comments on an earlier version of the manuscript.
Ethics statement
The study was approved by the University of Western Australia Animal Ethics Committee (permit number: RA/3/100/1050).
Data accessibility
Data are available at doi:10.5061/dryad.n687q.
Funding statement
This research was supported by a Marie Curie IOF within the 7th European Community Framework Programme and an ECR grant from UWA to C.G.
References
- 1.Velando A, Torres R, Alonso-Alvarez C. 2008. Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. Bioessays 30, 1212–1219. ( 10.1002/bies.20838) [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt K. 2007. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82, 375–393. ( 10.1086/522811) [DOI] [PubMed] [Google Scholar]
- 3.Aitken RJ, Krausz C. 2001. Oxidative stress, DNA damage and the Y chromosome. Reproduction 122, 497–506. ( 10.1530/rep.0.1220497) [DOI] [PubMed] [Google Scholar]
- 4.Jones TM, Elgar MA. 2004. The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc. R. Soc. Lond. B 271, 1311–1318. ( 10.1098/rspb.2004.2723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White J, Wagner RH, Helfenstein F, Hatch SA, Mulard H, Naves LC, Danchin E. 2008. Multiple deleterious effects of experimentally aged sperm in a monogamous bird. Proc. Natl Acad. Sci. USA 105, 13 947–13 952. ( 10.1073/pnas.0803067105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan CKW, Pizzari T, Wigby S. 2013. Parental age, gametic age, and inbreeding interact to modulate offspring viability in Drosophila melanogaster. Evolution 67, 3043–3051. ( 10.1111/evo.12131) [DOI] [PubMed] [Google Scholar]
- 7.Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB. 2008. The evolutionary ecology of pre- and post-meiotic sperm senescence. Trends Ecol. Evol. 23, 131–140. ( 10.1016/j.tree.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 8.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 9.Denk AG, Holzmann A, Peters A, Vermeirssen ELM, Kempenaers B. 2005. Paternity in mallards: effects of sperm quality and female sperm selection for inbreeding avoidance. Behav. Ecol. 16, 825–833. ( 10.1093/beheco/ari065) [DOI] [Google Scholar]
- 10.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. ( 10.1016/S0960-9822(03)00939-4) [DOI] [PubMed] [Google Scholar]
- 11.Houde AE. 1997. Sex, color and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 12.Blount JD, Møller AP, Houston DC. 2001. Antioxidants, showy males and sperm quality. Ecol. Lett. 4, 393–396. ( 10.1046/j.1461-0248.2001.00255.x) [DOI] [Google Scholar]
- 13.Gasparini C, Devigili A, Dosselli R, Pilastro A. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3, 4940–4953. ( 10.1002/ece3.870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman MM, Kelley JL, Evans JP. 2013. Condition-dependent expression of pre- and postcopulatory sexual traits in guppies. Ecol. Evol. 3, 2197–2213. ( 10.1002/ece3.632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans JP. 2011. Patterns of genetic variation and covariation in ejaculate traits reveal potential evolutionary constraints in guppies. Heredity 106, 869–875. ( 10.1038/hdy.2010.130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neff BD, Pitcher TE, Ramnarine IW. 2008. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol. Ecol. 17, 2975–2984. ( 10.1111/j.1365-294X.2008.03816.x) [DOI] [PubMed] [Google Scholar]
- 17.Birkhead TR, Fletcher F. 1995. Male phenotype and ejaculate quality in the zebra finch Taeniopygia guttata. Proc. R. Soc. Lond. B 262, 329–334. ( 10.1098/rspb.1995.0213) [DOI] [PubMed] [Google Scholar]
- 18.Helfenstein F, Losdat S, Møller AP, Blount JD, Richner H. 2010. Sperm of colourful males are better protected against oxidative stress. Ecol. Lett. 13, 213–222. ( 10.1111/j.1461-0248.2009.01419.x) [DOI] [PubMed] [Google Scholar]
- 19.Pike TW, Blount JD, Lindstrom J, Metcalfe NB. 2010. Dietary carotenoid availability, sexual signalling and functional fertility in sticklebacks. Biol. Lett. 6, 191–193. ( 10.1098/rsbl.2009.0815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siva-Jothy MT. 2000. The young sperm gambit. Ecol. Lett. 3, 172–174. ( 10.1046/j.1461-0248.2000.00146.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at doi:10.5061/dryad.n687q.