Abstract
Parasites, by altering the nutritional and energetic state of their hosts, can significantly alter their foraging behaviour. In honeybees, an infection with Nosema ceranae has been shown to lower the energetic state of individual bees, bringing about changes in behaviours associated with foraging. Comparing the foraging trip times, hive times in between trips, and the crop contents of uninfected and infected foragers as they depart on foraging trips and return from them, this study examined how any differences in these variables influence alternative foraging currencies. The results show that infected bees take longer foraging trips, spend shorter time in the hive between successive trips and bring back less sugar from each trip. These changes have a stronger adverse effect on their efficiency of energetic gain as compared with their rate of energetic gain, which has important implications for individual and colony life history.
Keywords: optimal foraging, currency, energetics, life history, honeybees, disease
1. Introduction
The foraging behaviour of an animal is fundamentally driven by its nutritional and energetic state. Parasites, by drawing nutrition and eliciting energetically expensive immune responses, can therefore alter the foraging behaviour of their hosts [1]. Behavioural alterations, including those related to foraging, as consequences of parasitism and disease have been extensively studied, but only rarely have these changes been explored from the perspective of an optimal foraging framework [2]. Optimal foraging models, which use appropriate currencies and cost–benefit functions based on several parameters and constraints to make quantitative predictions about how an animal forages, can therefore be powerful tools to evaluate how the fitness of the host can be compromised by a parasitic disease that alters its foraging behaviour. Such changes in foraging, in addition to having indirect negative effects on host fitness, might also in turn affect parasite fitness by influencing their transmission dynamics.
Parasites and pathogens have been routinely linked to the recent decline in honeybee populations [3], but their negative role has generally been considered only from a direct, pathological viewpoint. Some studies have however shown parasitic infections, such as one with the microsporidian Nosema ceranae, have a negative impact on the foraging behaviour of honeybees [4–7], which could be related to a significant energetic stress in the infected individuals [8] and its influence on a variety of specific behaviours [9–11]. However, how these changes translate to individual- and colony-level effects in terms of nutritional budgets and life-history patterns are not well known. Central place foragers such as honeybees with a fixed lifetime flight cost budget are generally predicted to maximize their efficiency of energetic gain, (Gain−Cost)/Cost rather than their net rate of energetic gain, (Gain−Cost)/Time, during foraging as this allows them to maximize their foraging lifespan [12–14]. The goal of this study was therefore to determine whether a parasitic infection can influence the foraging behaviour in terms of what is predicted by these two alternative foraging currencies.
2. Material and methods
An observation hive was assembled with adult bees and brood from a source colony, which was also used to supply the observation hive with 500 one-day-old bees every other week. Capped brood was extracted 1–2 days before they were due to hatch and kept in an incubator maintained at 32°C. Newly emerged bees were tagged with unique number tags and individually fed, half of them with 30 µl of sucrose solution and the other half with 30 µl of sucrose solution containing N. ceranae spores at a concentration of 1 × 106 ml−1, before being introduced into the observation hive.
Behavioural observations were conducted on the observation hive for 4 h, alternating between morning and afternoon sessions, 4 days a week, in those weeks in which new bees were not introduced into the hive. Behavioural sampling consisted of watching the entrance of a hive and recording the departure and arrival times of tagged foragers. Once a tagged forager had a record of successive departure and arrival times from which at least five trip times and between-trips hive times could be calculated, she was opportunistically captured while she was either departing on a foraging flight or returning from one. The captured bees were euthanized and the crop content of each bee was collected on a coverslip by squeezing the thorax. The volume of the contents was measured with a graduated microcapillary tube and the sucrose concentration was determined with a hand-held refractometer [15]. The bee was then dissected to look for the presence of N. ceranae spores in the gut and classify her status as to whether being uninfected or infected.
The experiment was conducted simultaneously with two observation hives, each with its own source colony, and observations alternating between the two hives every week. No significant main or interactive effect of the colony was found during initial data analysis and it was therefore dropped as a factor from subsequent analyses (see the electronic supplementary material for full analysis).
3. Results
Whether a forager was infected or not had a significant influence on the duration of her foraging trip (one-way ANOVA, F1,237 = 126.69, p < 0.001) and the time she spent inside the hive in between two successive trips (F1,237 = 26.50, p < 0.001, figure 1). Infected foragers took longer foraging trips and spent a shorter time in the hive in between two successive trips. The amount of sucrose in the crop of a departing or a returning forager was calculated using the volume and sucrose concentration of nectar in the extracted crop contents [15]. The amount of sucrose in the crop was found to be significantly influenced by whether a forager was infected or not (F1,235 = 8.17, p = 0.005) and by whether she was captured during her departure or return flight (F1,235 = 80.03, p < 0.001), with no significant interaction between these two factors. While returning foragers not surprisingly had a significantly higher amount of sucrose in their crops than departing ones, more interestingly, uninfected foragers returned with a significantly higher amount of sugar in their crops than infected foragers (t97 = 2.57, p = 0.01, figure 1) even though there was no significant difference between the crop sugar content of the two groups while departing (t63 = 2.01, p = 0.05). The difference in the amount of sugar brought back by the two types of foragers resulted from uninfected foragers bringing back a significantly higher volume (t120 = 2.14, p = 0.03; electronic supplementary material, figure S1) of nectar with a significantly higher sucrose concentration (t103 = 2.05, p = 0.04).
Figure 1.
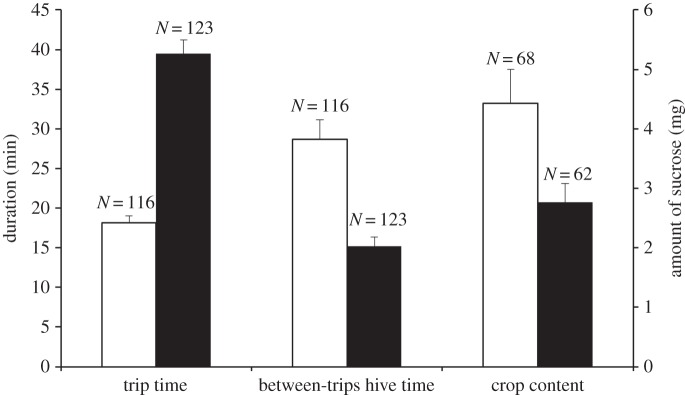
Foraging parameters—time spent in a foraging trip, time spent in the hive between two successive trips and the foraging return from each trip—for uninfected and infected foragers, with data consisting of mean ± s.e.m. Unfilled bars, control; filled bars, infected.
Using the mean values for the amount of sucrose brought back (W), trip time (τ0), time spent in the hive between two trips (T0), calorific value of sucrose (c), metabolic rate of forager in the hive (aT), metabolic rate of unloaded forager (a0) and the linear increase in metabolic rate with load (a) in the framework used in earlier studies [14,15], I calculated the two foraging currencies, net rate of energetic gain and efficiency of energetic gain for uninfected and infected foragers as
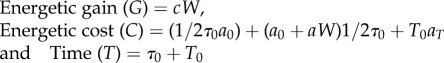 |
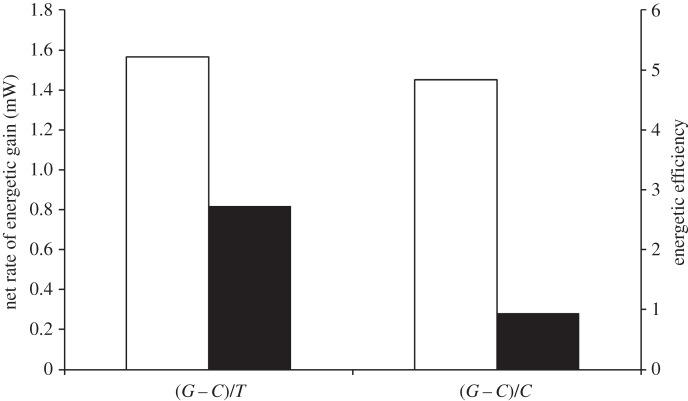
In the absence of any data regarding the flight distance and patch behaviour of these free foraging bees, these calculations make the reasonable assumption that the trip cost almost entirely consists of two parts, the bee flying unloaded for half the trip time and flying loaded for the other half with the crop sugar mass detected on her return to the hive. These calculations show that for infected foragers, the rate of energetic gain drops to about 1/2 while the efficiency drops to about 1/5 of its value in uninfected foragers (figure 2).
Figure 2.
Foraging currencies—rate and efficiency of energetic gain for uninfected and infected foragers. Unfilled bars, control; filled bars, infected.
4. Discussion
These results show that an infection with N. ceranae causes important differences to the foraging behaviour of honeybees. The longer foraging trips and the shorter hive time in between two successive trips seen for infected bees could both be consequences of their lower energetic state [8], which supports earlier assertions that the foraging behaviour of individuals even in a eusocial group is still partly driven by their own energetic demands [10]. It is also interesting that the longer duration spent on a foraging trip does not translate to a higher foraging return for infected bees, which could result from a reduced ability in locating quality resources. The likely factors underlying this reduction are the longer time spent by such bees in orientation and flight [4,5,7] and the learning impairment seen in both infected [16] and energetically stressed bees [11], even more reinforced by the fact that any precocious foraging by infected bees [6] would have alleviated such reductions.
If the foraging trips for infected bees are longer but their returns are lower, what does it mean for the two foraging currencies? The results show that infected bees incur a stronger negative effect on their efficiency of energetic gain than in their rate of energetic gain, which is likely to translate into a stronger negative impact on lifespan at the individual level and therefore a lower long-term colony energy budget. Note that the calculations here do not assume any difference in the metabolic rates between infected and uninfected foragers, which are most likely to exist and exacerbate these differences in terms of the two currencies even further. As longer trip times will always result in a higher loss of efficiency than in the rate of energetic gain, whether the smaller loads carried by infected bees is an adaptive response to mitigate these increased costs of longer trips is an interesting question. The decrease in net energetic gain seen here is in contrast to the results from an earlier study, which did not find a significant effect of infection on foraging gains made by infected foragers [17]. This emphasizes that travel costs make the largest impact on these foraging currencies, and studies in controlled environments [12–14,17] overestimate foraging gains and diminish any differences that might exist between foragers at different physiological states, especially in nutritionally challenging environments [18].
If infected individuals forage differently, it has important implications not only for host fitness but also for the transmission potential of the pathogen, a factor that has traditionally not been an important part of infectious disease models. The foraging biology of an animal can not only influence its exposure and therefore its susceptibility as a potential host, but can also determine its effectiveness as an infectious individual acting as a transmitting agent. The fact that foraging behaviour has major effects on community-level interactions among different species and that this has important relevance for disease ecology has only recently begun to be appreciated [19,20].
The results of this study most importantly suggest that an energetic shortfall in honeybee foragers due to a parasitic infection or otherwise can alter their foraging pattern in a manner that in turn might also lead to a lower lifespan, thereby having significant effects on both individual and colony life history. A disease can thus have an indirect but substantive demographic effect that can lead to a strong decline in the population size of a honeybee colony, a phenomenon which has recently attracted a lot of attention and has often been characterized as the sudden loss of bees from colonies and their resulting collapse.
Supplementary Material
Supplementary Material
Acknowledgements
D.N. thanks Amanda Stammer for helping to collect the data with Keziah Katz, and Arathi Seshadri for rescuing the figures.
Funding statement
The study was made possible by a NSF CAREER award to D.N.
References
- 1.Moore J. 2002. Parasites and the behavior of animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Lozano GA. 1991. Optimal foraging theory: a possible role for parasites. Oikos 60, 391–395. ( 10.2307/3545084) [DOI] [Google Scholar]
- 3.Ratnieks FLW, Carreck NL. 2010. Clarity on honey bee collapse? Science 327, 152–153. ( 10.1126/science.1185563) [DOI] [PubMed] [Google Scholar]
- 4.Kralj J, Fuchs S. 2010. Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41, 21–28. ( 10.1051/apido/2009046) [DOI] [Google Scholar]
- 5.Dussaubat C, Maisonnasse A, Crauser D, Beslay D, Costagliola G, Soubeyrand S, Kretzchmar A, Le Conte Y. 2013. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invert. Pathol. 113, 42–51. ( 10.1016/j.jip.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 6.Goblirsch M, Huang ZY, Spivak M. 2013. Physiological and behavioral changes in honey bees Apis mellifera induced by Nosema ceranae infection. PLoS ONE 8, e58165 ( 10.1371/journal.pone.0058165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf S, McMahon DP, Lim KS, Pull CD, Clark SJ, Paxton RJ, Osborne JL. 2014. So near and yet so far: Harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE 9, e103989 ( 10.1371/journal.pone.0103989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayack C, Naug D. 2010. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 56, 1572–1575. ( 10.1016/j.jinsphys.2010.05.016) [DOI] [PubMed] [Google Scholar]
- 9.Mayack C, Naug D. 2011. A changing but not an absolute energy budget dictates risk-sensitive behaviour in the honeybee. Anim. Behav. 82, 595–600. ( 10.1016/j.anbehav.2011.06.022) [DOI] [Google Scholar]
- 10.Mayack C, Naug D. 2013. Individual energetic state can prevail over social regulation of foraging in honeybees. Behav. Ecol. Sociobiol. 67, 929–936. ( 10.1007/s00265-013-1517-6) [DOI] [Google Scholar]
- 11.Jaumann S, Scudelari R, Naug D. 2013. Energetic cost of learning and memory can cause cognitive impairment in honeybees. Biol. Lett. 9, 20130149 ( 10.1098/rsbl.2013.0149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid-Hempel P, Kacelnik A, Houston AI. 1985. Honeybees maximize efficiency by not filling their crop. Behav. Ecol. Sociobiol. 17, 61–66. ( 10.1007/BF00299430) [DOI] [Google Scholar]
- 13.Seeley TD. 1986. Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav. Ecol. Sociobiol. 19, 343–354. ( 10.1007/BF00295707) [DOI] [Google Scholar]
- 14.Schmid-Hempel P, Wolf T. 1988. Foraging effort and life span of workers in a social insect. J. Anim. Ecol. 57, 509–521. ( 10.2307/4921) [DOI] [Google Scholar]
- 15.Dafni A, Kevan PG, Husband BC. 2005. Practical pollination biology. Cambridge, UK: Enviroquest. [Google Scholar]
- 16.Kralj J, Brockmann A, Fuchs S, Tautz J. 2007. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Comp. Physiol. A 193, 363–370. ( 10.1007/s00359-006-0192-8) [DOI] [PubMed] [Google Scholar]
- 17.Ellis A, Delaplane K. 2009. Individual forager profits in Apis mellifera unaffected by a range of colony Varroa destructor densities. Insectes Soc. 56, 419–424. ( 10.1007/s00040-009-0040-2) [DOI] [Google Scholar]
- 18.Naug D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142, 2369–2372. ( 10.1016/j.biocon.2009.04.007) [DOI] [Google Scholar]
- 19.Fels D. 2005. The effect of food on microparasite transmission in the waterflea Daphnia magna. Oikos 109, 360–366. ( 10.1111/j.0030-1299.2005.13812.x) [DOI] [Google Scholar]
- 20.Hall SR, Becker LS, Becker C, Duffy MA, Tessier AJ, Cáceres CE. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol. Lett. 10, 207–218. ( 10.1111/j.1461-0248.2007.01011.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.