Abstract
Inbred individuals and populations are predicted to suffer from inbreeding depression, especially in times of stress. Under natural conditions, organisms are exposed to more than one stressor at any one time, highlighting the importance of stress resistance traits. We studied how inbreeding- and immunity-related traits are correlated under different dietary conditions in the ant Formica exsecta. Its natural diet varies in the amount and nature of plant secondary compounds and the level of free radicals, all of which require detoxification to maintain organismal homeostasis. We found that inbreeding decreased general antibacterial activity under dietary stress, suggesting inbreeding-related physiological trade-offs.
Keywords: inbreeding, trade-offs, immunity, reactive oxygen species, diet, Formica exsecta
1. Introduction
Life-history trade-offs have been extensively studied in terms of individual performance, but associated processes at the molecular level are less well understood. The gene networks underpinning molecular stress and their impact on trade-offs between reproduction and survival [1] are shaped through the selection on stress response traits. Inbreeding, in conjunction with genetic drift and selection, reduces genetic variation and can have negative effects on fitness via altering life-history traits, as well as performance and survival [2,3]. Of special interest are immunity-related traits, as defence against parasites is paramount in order to maintain fitness. Immune functions in insects are regulated by several regulatory pathways, the genes of which are well charted [4], but immunity can also be enhanced by diet [5]. Thus, immunocompetence entails complex trade-offs with many other physiological responses [6], and evidence suggests that inbred animals suffer from immunodeficiency [7–9]. The genetic and physiological underpinnings of immune functions have been extensively studied in laboratory-based model organisms, including insects. Yet the extent to which inbreeding influences stress resistance and immunity, and trade-offs between these in wild populations has not received due attention [10].
Immune defences are tightly linked to other stress responses, such as release of significant amounts of free radicals (reactive oxygen species, ROS) upon infection. ROS are considered highly effective against many pathogens [11]. However, they are also a major source of oxidative stress, thereby damaging DNA, proteins and lipids [12,13]. Accumulation of this type of damage causes cellular senescence and can influence organismal ageing and lifespan. Therefore, while release of ROS is an effective part of immunity, it carries a cost, which needs to be attenuated by matching levels of antioxidants. Another source of ROS is diet, as release of free radicals is a part of plant responses to herbivory [14]. Inbreeding has been suggested to confer impaired stress resistance, in terms of both immunity and antioxidant responses. As a result, trade-offs between these two complementary responses may escalate costs to inbred individuals [7]. Alternatively, some form of compensation may occur.
Here, we test whether inbreeding is associated with reduced stress resistance and a compromised immune system in the ant Formica exsecta. We achieve this by analysing the effect of free radicals in the diet on the survival of foragers and by testing the effect of diet on the expression of immune-related and oxidative stress-related genes. We show that inbreeding is an important predictor of stress resistance.
2. Material and methods
(a). Animals
Our study population of F. exsecta, located on the Hanko peninsula, Southwestern Finland, has been monitored since 1994, and data on colony kin structure, birth and death collected on a yearly basis [8,15,16] (see electronic supplementary material, Materials and methods for additional details).
In spring 2012, we collected ca 400 workers from each of 21 single-queen colonies in the field. These workers were of the same age, given that new workers are produced in yearly discrete cohorts and workers live ca 1 year [17]. Upon collection, the workers were transferred to the laboratory where they were placed in plastic boxes (25 × 15 × 10 cm) lined with Fluon (Whitford) to prevent the ants from escaping. Nests were kept at room temperature (20–25°C), and before the start of the experiments the ants were fed Bhatkar–Whitcomb [18] diet ad libitum, and moistened daily. The ants were kept in these conditions for 30 days to allow them to habituate to their new environment before the experiment started. The actual experiment was carried out in the laboratory at a constant temperature of 23°C.
(b). Experimental design
To assess whether the presence of dietary ROS influences the basal rate of immune defence, we used two dietary regimes, one in which the ants received Bhatkar–Whitcomb diet supplemented with 4% H2O2 (Merck), and a control group, which received standard Bhatkar–Whitcomb diet. At the start of the experiment, the ants were transferred to round jars (Ø 7 cm, H 5 cm), the bottoms of which were covered with plaster (144 ants per colony with 36 ants per jar distributed across four jars). An Eppendorf tube with water and a cotton plug supplied moisture, and fresh food was provided daily ad libitum according to treatment. Dead ants were counted and removed daily. After 4 days, samples were collected from all 21 colonies as follows: three sets of two ants per colony and treatment (taken randomly from the four jars) were homogenized in 40 µl of ice cold phosphate-buffered saline for measurement of lytic activity, and three sets of three ants per treatment and colony (taken randomly from the four jars) were fixed in 200 µl of RNALater (Ambion) and cut into small pieces to assess the gene expression. The number of ants used in the assays was standardized to allow comparisons between replicates. To avoid degradation of the enzymes, the formic acid gland was removed by cutting off the two terminal abdominal segments before fixing the samples for the lytic assay. All samples were stored at −80°C until further use (electronic supplementary material, Material and methods).
We studied the expression of 11 genes involved in immunity and oxidative stress: defensin, NFκB, Toll, lysozyme C, prophenoloxidase, vitellogenin 1, vitellogenin-like protein 4, dual oxidase 1, peroxidase, sodium dismutase 1 and 2, respectively. Defensin, lysozyme C and prophenoloxidase are effector molecules, actively interacting with pathogens [4], whereas vitellogenins [19] carry both storage- and immunity-related functions. Dual oxidase 1 is released in the gut tissue upon bacterial infection [20], whereas NFκB is a transcription factor [11] and Toll is a membrane-bound receptor in the signalling cascade [4]. Peroxidase and sodium dismutases were selected as genes encoding for antioxidant enzymes [21,22].
(c). Data analysis
All analyses were conducted in R v. 3.1.0. [23]. Survival was analysed using parametric survival regression models (Survreg function of the survival package), including HL (homozygosity by locus) as a continuous variable and treatment as a factor. Pseudoreplication was accounted for by adding Colony as a random effect using the frailty command [24]. Lytic activity was analysed using a generalized linear mixed model (GLMM), including HL and treatment, as well as the interaction between the two. Colony was added as a random effect and the model was specified with a Poisson distribution. The effect of inbreeding and treatment on gene expression was tested with MANOVA, using the log transformed dCT values of each gene as dependent variables, and treatment and HL, as well as the interaction between the two, as factors. The effects of treatment on each single gene were tested with post hoc ANOVAs using the summary.aov function. As 11 genes were compared in this way, we corrected our significance level to α = 0.0045.
3. Results
The presence of free radicals in the diet significantly reduced worker survival (Survreg, diet, z = −3.921, p < 0.001, figure 1), but no effect of inbreeding on mortality was detected (Survreg, HL, z = −1.169, p = 0.24), nor did inbred ants react differently to ROS-feeding (Survreg, diet × HL, z = 0.753, p = 0.45). Lytic activity was significantly lower in the group that received free radicals in the diet (GLMM, diet, z = −3.12, p = 0.002). Furthermore, lytic activity increased significantly with the level of inbreeding in the control group, but not the group that received free radicals in the diet (GLMM, diet × HL, z = −2.786, p = 0.005, figure 2). None of the genes was affected by treatment or HL. This is not due to the conservative nature of Bonferroni correction, as the only significant value we obtained before correcting for treatment was for dual oxidase (DUOX1), which showed a weak significant effect of diet (F = 5.02, p = 0.032, electronic supplementary material, table S1).
Figure 1.
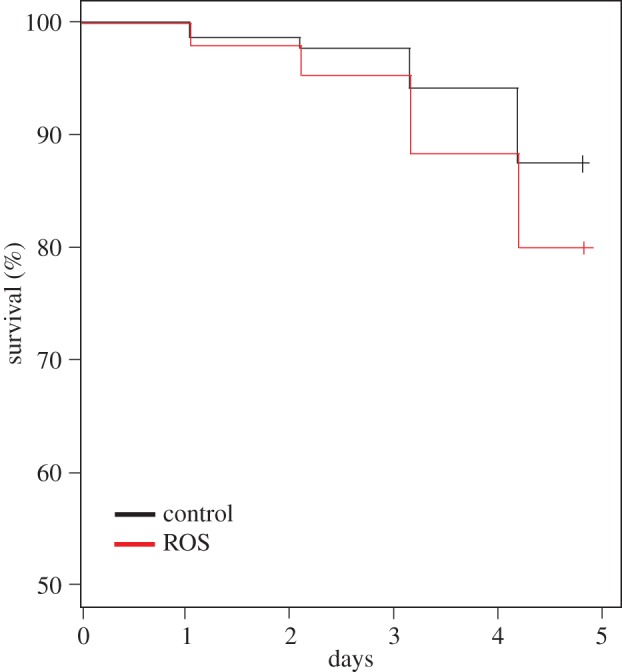
The effect of inbreeding and dietary ROS on mortality. Ants fed with ROS died faster than ants that received control diet. (Online version in colour.)
Figure 2.
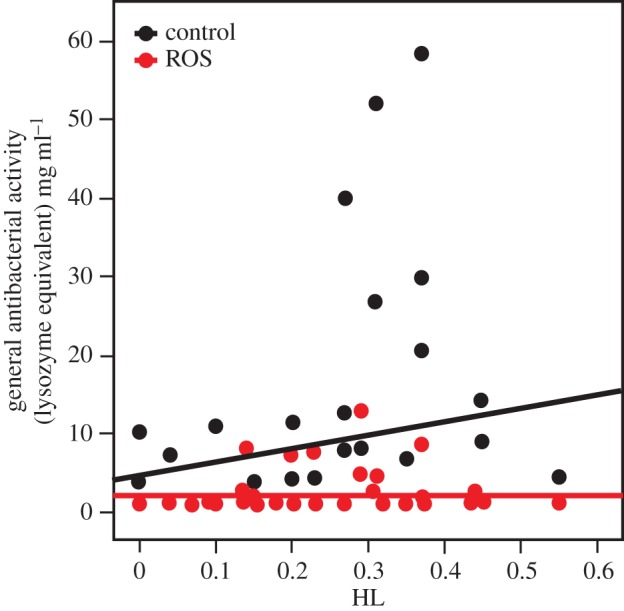
The effect of inbreeding and dietary ROS on immunity. Inbred ants show higher levels of lysozyme-like activity on control diet, but not when fed dietary ROS. (Online version in colour.)
4. Discussion
Here, we demonstrate differential responses to dietary stress in inbred and outbred colonies of F. exsecta. Supplementing the ants' diet with free radicals led to increased mortality, regardless of the level of inbreeding. Furthermore, we show that more inbred individuals have higher steady-state lytic activity and are thus likely to pay a higher cost in terms of production and maintenance of immunity [6] under parasite-free conditions. Following oxidative challenge, inbred individuals downregulated immune functions. This suggests that when exposed to a dietary stressor, highly inbred individuals may be more vulnerable to infections than less inbred ones. Thus, vital functions and stress responses can be traded-off against each other, as shortcomings in one capacity (inbreeding) can be partly compensated by regulating another (immune defence).
Of the 11 immune and antioxidant genes studied, none showed differential expression upon oxidative challenge nor was influenced by the level of inbreeding. The most plausible explanation for this could be that we did not target the right genes. Alternatively, compensatory responses could be responsible for dealing with oxidative stress. Full transcriptome sequencing could give a more precise answer to the question of which genes are affected by inbreeding and/or oxidative stress. Regardless of gene expression, both inbreeding and oxidative challenge increased lytic activity. Higher lytic activity in inbred animals may be due to the presence of more pathogens in inbred colonies or reduced physiological homeostasis [8]. It is also possible that more inbred individuals are less able to cope with oxidative stress, as they are observed to be less physically active in general [25].
Here, we provide evidence that inbred individuals may be less able to handle trade-offs associated with two central life-history traits, immune functions and dietary stress, than outbred ones [1]. The steady-state lytic activity correlates positively with inbreeding, indicating that costs associated with innate responses depend on the genetic heterozygosity of the organism.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sini Vuorensyrjä for help.
Funding statement
We thank the Academy of Finland (grants nos 251337, 6303369) and University of Helsinki for funding.
References
- 1.Flatt T, Heyland A. 2011. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Fox CW, Stillwell RC, Wallin WG, Curtis CL, Reed DH. 2011. Inbreeding-environment interactions for fitness: complex relationships between inbreeding depression and temperature stress in a seed-feeding beetle. Evol. Ecol. 25, 25–43. ( 10.1007/s10682-010-9376-3) [DOI] [Google Scholar]
- 3.Reed DH, Fox CW, Enders LS, Kristensen TN. 2012. Inbreeding–stress interactions: evolutionary and conservation consequences. Ann. NY Acad. Sci. 1256, 33–48. ( 10.1111/j.1749-6632.2012.06548.x) [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743. ( 10.1146/annurev.immunol.25.022106.141615) [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Friman V-P, Laakso J, Mappes J. 2012. Interactive effects between diet and genotypes of host and pathogen define the severity of infection. Ecol. Evol. 2, 2347–2356. ( 10.1002/ece3.356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551. ( 10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- 7.Franke K, Fischer K. 2013. Effects of inbreeding and temperature stress on life history and immune function in a butterfly. J. Evol. Biol. 26, 517–528. ( 10.1111/jeb.12064) [DOI] [PubMed] [Google Scholar]
- 8.Vitikainen E, Sundström L. 2011. Inbreeding and caste-specific variation in immune defence in the ant Formica exsecta. Behav. Ecol. Sociobiol. 65, 899–907. ( 10.1007/s00265-010-1090-1) [DOI] [Google Scholar]
- 9.Whitehorn PR, Tinsley MC, Brown MJF, Darvill B, Goulson D. 2011. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc. R. Soc. B 278, 1195–1202. ( 10.1098/rspb.2010.1550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundström L, Seppä P, Pamilo P. 2005. Genetic population structure and dispersal patterns in Formica ants—a review. Ann. Zool. Fennici 42, 163–177. ( 10.1007/BF00172934) [DOI] [Google Scholar]
- 11.Ryu J-H, et al. 2006. An essential complementary role of NF-κB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 25, 3693–3701. ( 10.1038/sj.emboj.7601233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745. ( 10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selman C, Blount JD, Nussey DH, Speakman JR. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570–577. ( 10.1016/j.tree.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 14.Kerchev PI, Fenton B, Foyer CH, Hancock RD. 2012. Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 35, 441–453. ( 10.1111/j.1365-3040.2011.02399.x) [DOI] [PubMed] [Google Scholar]
- 15.Vitikainen E, Haag-Liautard C, Sundström L. 2011. Inbreeding and reproductive investment in the ant Formica exsecta. Evolution 65, 2026–2037. ( 10.1111/j.1558-5646.2011.01273.x) [DOI] [PubMed] [Google Scholar]
- 16.Sundström L, Keller L, Chapuisat M. 2003. Inbreeding and sex-biased gene flow in the ant Formica exsecta. Evolution 57, 1552–1561. ( 10.1111/j.0014-3820.2003.tb00363.x) [DOI] [PubMed] [Google Scholar]
- 17.Bargum K, Sundström L. 2007. Multiple breeders, breeder shifts and inclusive fitness returns in an ant. Proc. R. Soc. B 274, 1547–1551. ( 10.1098/rspb.2007.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatkar A, Whitcomb WH. 1970. Artificial diet for rearing various species of ants. Florida Entomol. 53, 229–232. ( 10.2307/3493193) [DOI] [Google Scholar]
- 19.Tong Z, Li L, Pawar R, Zhang S. 2010. Vitellogenin is an acute phase protein with bacterial-binding and inhibiting activities. Immunobiology 215, 898–902. ( 10.1016/j.imbio.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 20.Ha E-M, Lee K-A, Seo YY, Kim S-H, Lim J-H, Oh B-H, Kim J, Lee W-J. 2009. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat. Immunol. 10, 949–57. ( 10.1038/ni.1765) [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. 2010. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327, 1644–1648. ( 10.1126/science.1184008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CY, Hsieh YS. 2014. Oxidative stress decreases in the trophocytes and fat cells of worker honeybees during aging. Biogerontology 15, 129–137. ( 10.1007/s10522-013-9485-9) [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 24.Reber A, Castella G, Christe P, Chapuisat M. 2008. Experimentally increased group diversity improves disease resistance in an ant species. Ecol. Lett. 11, 682–689. ( 10.1111/j.1461-0248.2008.01177.x) [DOI] [PubMed] [Google Scholar]
- 25.Ugelvig LV, Kronauer DJC, Schrempf A, Heinze J, Cremer S. 2010. Rapid anti-pathogen response in ant societies relies on high genetic diversity. Proc. R. Soc. B 277, 2821–2828. ( 10.1098/rspb.2010.0644) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


