Abstract
BACKGROUND
An exaggerated morning blood pressure surge (MBPS) may be associated with stroke and other cardiovascular events, but the threshold at which an MBPS becomes pathological is unclear. This study aimed to systematically review the existing literature and establish the most appropriate definition of pathological MBPS.
METHODS
A MEDLINE search strategy was adapted for a range of literature databases to identify all prospective studies relating an exaggerated MBPS to cardiovascular endpoints. Hazard ratios (HRs) were extracted and synthesized using random-effects meta-analysis.
RESULTS
The search strategy identified 2,964 unique articles, of which 17 were eligible for the study. Seven different definitions of MBPS were identified; the most common was a prewaking surge (mean blood pressure for 2 hours after wake-up minus mean blood pressure for 2 hours before wake-up; n = 6 studies). Summary meta-analysis gave no clear evidence that prewaking MBPS (defined by a predetermined threshold: >25–55mm Hg) was associated with all cardiovascular events (n = 2 studies; HR = 0.94, 95% confidence interval (CI) = 0.39–2.28) or stroke (n = 2 studies; HR = 1.26, 95% CI = 0.92–1.71). However, using a continuous scale, which has more power to detect an association, there was evidence that a 10 mm Hg increase in MBPS was related to an increased risk of stroke (n = 3 studies; HR = 1.11, 95% CI = 1.03–1.20).
CONCLUSIONS
These findings suggest that when measured and analyzed as a continuous variable, increasing levels of MBPS may be associated with increased risk of stroke. Large, protocol-driven individual patient data analyses are needed to accurately define this relationship further.
Keywords: ambulatory blood pressure monitoring, blood pressure, cardiovascular diseases, cardiovascular disease risk factors, circadian rhythm, hypertension, stroke.
Cardiovascular disease is the largest cause of morbidity and mortality worldwide.1 An exaggerated morning blood pressure surge (MBPS), ascertained using ambulatory blood pressure monitoring, is thought to be a risk factor for cardiovascular disease events occurring in the morning.2,3 This assumption is based on a number of prospective studies assessing the association between MBPS and subsequent cardiovascular disease.4–8 However, the prognostic value of MBPS for cardiovascular disease has been questioned, with more recent studies unable to reproduce the findings of earlier work.9,10
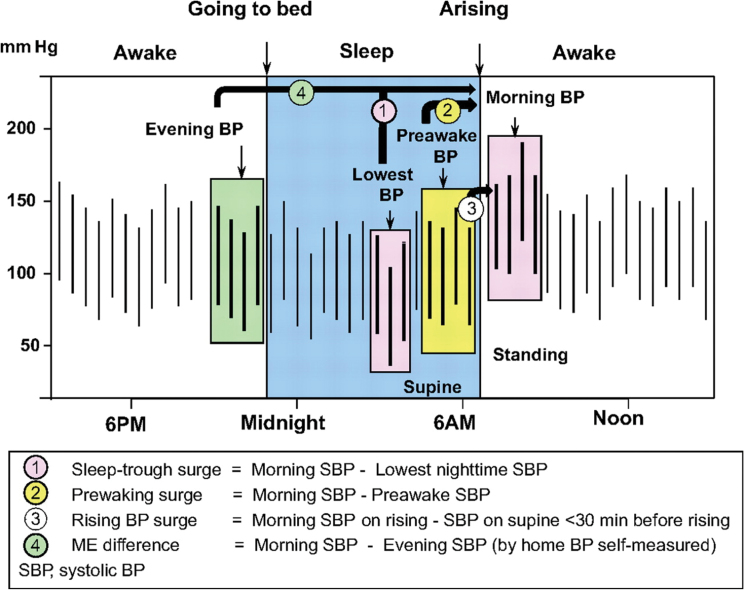
One possible cause of this disagreement is the many different definitions and thresholds used to define the MBPS in previous studies. For instance, MBPS is commonly defined as the sleep-trough surge, calculated by subtracting the morning blood pressure (mean of 4 readings over 2 hours just after wake-up) from the lowest nocturnal blood pressure (mean of 3 readings centred around the lowest nighttime blood pressure) (Figure 1).2,7 However, it has also been defined as the prewaking surge (morning blood pressure minus the 4 readings over 2 hours before waking)6,8 and the rising blood pressure surge (single morning blood pressure reading upon rising minus a single blood pressure reading 30 minutes before waking)11 among a variety of different definitions (Figure 1).12
Figure 1.
Definitions of morning, nighttime, and evening blood pressure measurements and morning blood pressure surge. This figure has been reproduced from Kario, K. (2010). Morning Surge in Blood Pressure and Cardiovascular Risk: Evidence and Perspectives. Hypertension, 56: 765–773. 2
Ambulatory blood pressure monitoring is becoming increasingly more common in routine clinical practice and has been recommended in the United Kingdom for the routine diagnosis of hypertension.13,14 With opportunities to assess and treat the MBPS increasing, it has never been more important to establish the prognostic significance of this phenomenon. This study therefore aimed to systematically review existing literature and establish the most appropriate definition of pathological MBPS, taking into account its relevance to cardiovascular disease morbidity and mortality and also the heterogeneity of the differing sample populations used in previous studies.
METHODS
Design
This study systematically reviewed all existing literature relating definitions of the MBPS to cardiovascular disease endpoints. The protocol and registration details of this study can be found online (http://www.crd.york.ac.uk/PROSPERO; registration number CRD42012002091).
Search strategy
A search strategy (see Supplementary Table S1) designed to capture all studies relating definitions of the MBPS to cardiovascular disease endpoints was developed for use with MEDLINE, and this was adapted to be run across the following additional databases: Cochrane (Wiley) CENTRAL Register of Controlled Trials, MEDLINE In Process (Ovid), EMBASE (Ovid), CINAHL (EBSCO), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), and Health Technology Assessment Database (HTA). The ZETOC (Mimas) database and Conference Proceedings Citation Index (ISI Web of Knowledge) were searched for conference proceedings and abstracts. In addition, the Current Controlled Trials metaRegister, NIHR Clinical Research Network Portfolio, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform were searched to locate any ongoing trials. Searches were carried out up to October 2013. To capture as broad a range of studies as possible, no study design filters were used, and no language or date limits were applied. In addition to searches of electronic databases, reference lists of studies included in the review were checked to identify further potentially relevant papers.
Selection of studies and inclusion criteria
Two reviewers (J.S. and J.H.) independently reviewed the titles and abstracts of potentially relevant articles for inclusion. Studies were selected for full document screening and data extraction provided they fulfilled the following inclusion criteria: (i) they were a prospective study; (ii) they defined and measured the MBPS; and (iii) they examined the relationship between MBPS and subsequent cardiovascular disease endpoints. All selected studies had to include at least 1 measurement of the MBPS in each study participant (at baseline, calculated from blood pressure measurements made using ambulatory or home blood pressure monitoring). No restrictions were made on the populations studied or the context in which MBPS was measured.
Data collection
Data were independently extracted from each included article by J.S. and J.H. Differences were resolved by consensus. Extracted data included any information about the sample population (e.g., patient demographics, mean blood pressure, dipping status, diagnosis of white coat or masked hypertension, prescribed medication, and history of cardiovascular disease and risk factors), the threshold value (if used), and definition of MBPS. Because the outcome was onset of cardiovascular disease subsequent to the measurement of MBPS, the suitable effect measure to quantify the association was a hazard ratio (HR).15 This compares the relative rate of cardiovascular disease in those with higher compared with lower MBPS values across the entire follow-up period. Where HRs were not reported directly, we used the methods of Parmar et al.16 to indirectly estimate them from other information available (such as a P value and number of events in each group). The data extraction sheet used is available in the Supplementary Methods.
Assessment of methodological quality
The methodological quality and risk of bias of individual studies was examined using the checklist described by Hayden et al. 17 for examining the quality of prognosis studies in systematic reviews, supplemented by further author-defined markers of methodological quality, including reporting of sampling and study follow-up.
Outcomes
The primary outcome of this review was to establish the most appropriate definition of MBPS that best describes its association with cardiovascular disease endpoints—specifically, all stroke events, all cardiovascular disease events, and all-cause mortality. Both analyses of MBPS thresholds (which define high and low MBPS values) and MBPS on a continuous scale were included.
Analysis
The characteristics and population demographics of each study were summarized using descriptive statistics. Log HR estimates and their confidence intervals (CIs) were synthesized into a random-effects meta-analysis using the method of DerSimonian and Laird.18 This method allows for between-study heterogeneity in the true HRs, and produces a pooled HR estimate and 95% CIs to summarize the prognostic association of MBPS for each outcome. There were insufficient studies to calculate prediction intervals, to perform meta-regression to explore causes of heterogeneity, or to investigate small study effects (potential publication bias).
RESULTS
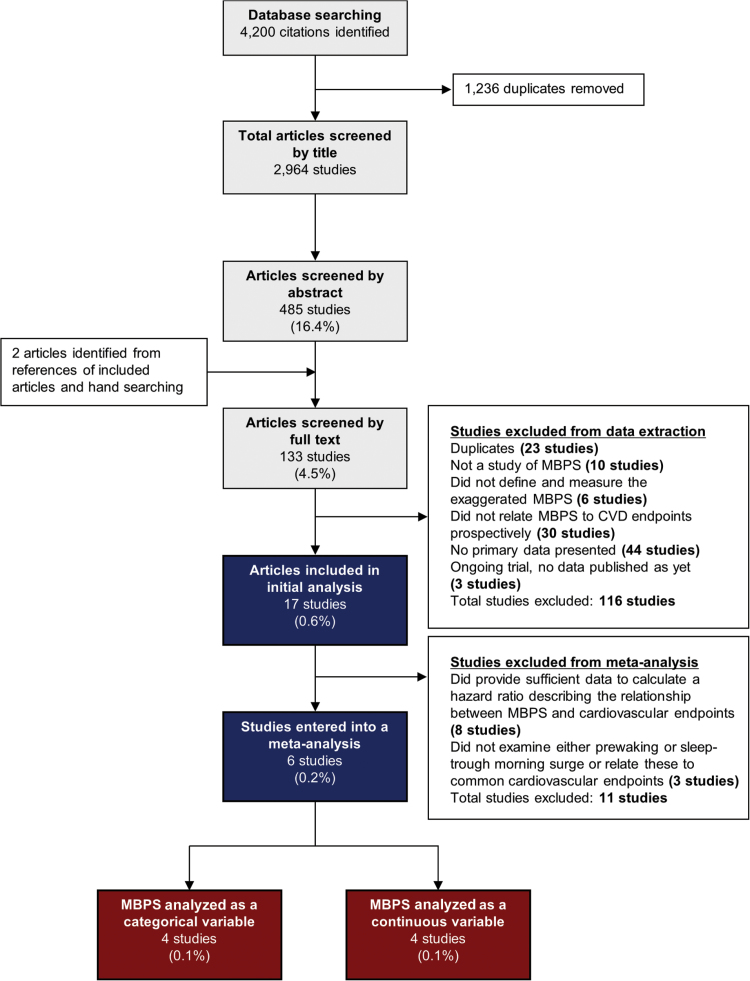
The search strategy identified 4,200 articles, of which 1,236 were duplicates. Of the remaining 2,964 articles, 133 (4.5%) were eligible for full-text screening, from which 17 (0.6%) were suitable for data extraction and included in the analysis (Figure 2). Included studies were conducted in 14 different countries and examined a total of 33,154 patients with a mean age of 60 years (Table 1). Studies differed according to sample size (42–11,291 patients), mean age (49–72 years), sex (32%–64% women), and the proportion of patients on blood pressure–lowering treatment (0%–76%) (Table 1). All studies recorded MBPS at baseline, and the majority (n = 11 studies) examined hypertensive patients in a secondary care setting. Patients were followed up for 37–137 months.
Figure 2.
Selection of studies to include in analysis of the effect of an exaggerated morning blood pressure surge (MBPS) on cardiovascular morbidity and mortality. Abbreviation: CVD, cardiovascular disease.
Table 1.
Population characteristics in individual studies examining the effect of an exaggerated morning blood pressure surge on cardiovascular morbidity and mortality
| Study | Country | Article type | Study setting | Study sample (hypertensive status) | Average follow-up period, mo | Sample size | Mean age, y (SD if available) | Sex (% female) | No. with hypertension (%) | No. on BP-lowering medication (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Amici et al. 5 | Italy | Full article | Secondary care | Hyper/normotension | 60 | 42 | 66 | 24 (57) | 32 (76) | 32 (76) |
| Amodeo et al. 26 | Brazil | Abstract | Not stated | Hyper/normotension | 60 | 633 | — | — | — | — |
| Dolan et al. 6 | Ireland | Abstract | Not stated | Hypertension | 64 | 11,291 | 55 | 5,984 (53) | 11,291 (100) | — |
| Gosse et al. 19 | France | Full article | Secondary care | Hypertension | 84 | 237 | 50 (12) | 76 (32) | 237 (100) | — |
| Gosse et al. 11 | France | Full article | Secondary care | Hypertension | 92 | 507 | 49 (12) | 183 (36) | 507 (100) | — |
| Hermida et al. 27 | Not stated | Abstract | Not stated | Not stated | 66 | 3,344 | 53 (15) | 1,626 (49) | — | — |
| Iqbal et al. 22 | UK | Full article | Secondary care | Hypertensionb | 65 | 245 | 60 (14) | 137 (56) | — | — |
| Israel et al. 10 | Israel | Full article | Secondary care | Hypertension | 78 | 2,627 | 57 | 1,419 (54) | 2,627 (100) | 1,550 (59) |
| Kario et al. 7 | Japan | Full article | Secondary care | Hypertension | 37c | 519 | 72 | — | 519 (100) | 285 (55) |
| Kario et al. 23 | Japan | Full article | Not stated | Hypertension | Not stated | 575 | — | — | 575 (100) | — |
| Li et al. 4 | Worldwidea | Full article | Hospital/university | General population | 137 | 5,645 | 53 (15) | 3,048 (54) | 2,314 (41) | 1,185 (21) |
| Metoki et al. 8 | Japan | Full article | Subject’s home | General population | 125 | 1,430 | 61 (11) | 915 (64) | — | 386 (27) |
| Metoki et al. 24 | Japan | Full article | Subject’s home | General population | 127 | 1,360 | 61 (11) | 870 (64) | — | 408 (30) |
| Nishinaga et al. 21 | Japan | Full article | Subject’s home | General population | 108 | 461 | 81 | 267 (58) | 272 (59) | 175 (38) |
| Reid et al. 25 | Australia | Abstract | Not stated | Hypertension | 66 | 712 | — | — | 712 (100) | — |
| Verdecchia et al. 9 | Italy | Full article | Secondary Care | Hypertension | 101 | 3,012 | 51 (12) | 1,386 (46) | 3,012 (100) | 0 (0) |
| Yano et al. 20 | Japan | Abstract | Not stated | Hypertension | 41 | 514 | 72 | 324 (63) | 514 (100) | — |
Abbreviation: SD, standard deviation.
aDenmark, Belgium, Russia, Italy, Poland, Japan, China, Uruguay.
bIncludes those with suspected hypertension.
cFollow-up in the control group (nonmorning surge); follow-up in the morning surge group was 41 months.
The methodological quality of each study is detailed in Table 2. Studies varied in methodological weakness (and reporting): all studies described how long patients were followed up and all but two described how the population was sampled.4–11,19–25 Only 9 of 17 studies reported satisfactory attrition rates,4,5,7,8,10,11,19,21,24 8 of 17 reported the planned sample size,4,7–9,19,21,22,26 and 11 of 17 reported how patients were selected for analysis.4,5,7–11,19,21,22,24 Reporting of outcome measures was generally good (n = 14 of 17 studies),4,11,19,21–25 but reporting of prognostic factor measurement and account of confounding was less satisfactory overall (n = 11 of 174,5,7–11,19–22 and 10 of 17 studies,4,6–11,19,24,27 respectively). Only 9 of 17 studies provided sufficient data to allow an HR to be calculated,4,6–11,24,25 which limited the number of studies that could be pooled in the meta-analysis. It should be noted that those studies included in the meta-analysis performed well in our assessment of methodological quality, other than Dolan et al.,6 which, as an abstract, lacked the sufficient detail required to properly examine its methodological strengths and weaknesses.
Table 2.
Assessment of methodological quality of included studies examining the effect of an exaggerated morning blood pressure surge on cardiovascular morbidity and mortality
| Study | Hayden et al. 17 checklist | Additional measures of methodological quality examined | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Study participation Study sample representative? |
Study attrition Does the data represent the sample? |
Prognostic factor measurement Is the prognostic factor (MBPS) sufficiently measured? |
Outcome measurement Is the outcome variable measured appropriately? |
Confounding measurement and account; Are potential confounders accounted for? |
Analysis Is the statistical analysis appropriate?a |
Sampling stated? | Selection method stated? | Planned sample size stated? | Period of follow-up given? | Was MBPS the primary focus of the study? | Was the study protocol published (or in the appendix)? | |
| Amici et al. 5 | No | Yes | Yes | Yes | Partly | No | Yes | Yes | No | Yes | Yes | No |
| Amodeo et al. 26 | Unsureb | Unsureb | Unsureb | Unsureb | Unsureb | Unsureb | No | No | Yes | Yes | Yes | No |
| Dolan et al. 6 | Unsureb | Unsureb | Unsureb | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No |
| Gosse et al. 19 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| Gosse et al. 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| Hermida et al. 27 | Unsureb | Unsureb | Unsureb | Unsureb | Yes | Yes | No | No | No | Yes | Yes | No |
| Iqbal et al. 22 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No |
| Israel et al. 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| Kario et al. 7 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Kario et al. 23 | No | No | No | Yes | No | No | Yes | No | No | Yes | No | No |
| Li et al. 4 | Yesc | Yes | Yes | Yes | Yes | Yes | Yes | Yesc | Yes | Yes | Yes | Yes 30 |
| Metoki et al. 8 | Yesc | Yes | Yes | Yes | Yes | Yes | Yes | Yesc | Yes | Yes | Yes | No |
| Metoki et al. 24 | Yesc | Yes | No | Yes | Yes | Yes | Yes | Yesc | Yes | Yes | No | No |
| Nishinaga et al. 21 | Unsure | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yesd | No |
| Reid et al. 25 | Unsureb | Unsureb | Unsureb | Yes | No | Yes | Yes | Unsureb | No | Yes | Yes | No |
| Verdecchia et al. 9 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Yano et al. 20 | Unsureb | Unsureb | Yes | Unsureb | Unsureb | Unsureb | Yes | Unsureb | No | Yes | Yes | No |
Abbreviation: MBPS, morning blood pressure surge.
aWas it possible to calculate a hazard ratio describing the relationship between MBPS and cardiovascular endpoints from data presented?
bAbstract with limited information.
cDetailed methods provided in another article. 30,41
dRelationship between morning blood pressure surge and cardiovascular disease endpoints was not the primary focus of the study.
A total of 7 different definitions of MBPS were assessed in the included studies (Tables 3 and 4). The most common were the sleep-trough surge (n = 8 studies),4,5,7–10,26,27 prewaking surge (n = 6 studies),4,6–10 and rising surge (n = 4 studies).10,11,19,20 Ten studies analyzed MBPS as a categorical variable using a predetermined threshold to define an exaggerated MBPS; 4 studies analyzed MBPS as a continuous variable; and 3 studies presented results for MBPS analyzed as both a categorical and continuous variable (Table 4). Thresholds for an exaggerated MBPS varied between studies from >12 to >153mm Hg (Table 4).
Table 3.
Definitions of morning blood pressure surge studied in included articles
| Morning blood pressure surge description | Definition |
|---|---|
| Sleep-trough surge | Morning blood pressure (average of 2 hours of readings after wake-up) minus the lowest nighttime reading (average of the lowest nighttime reading and the 2 adjacent readings before and after) |
| Prewaking surge | Morning blood pressure (average of 2 hours of readings after wake-up) minus the pre-awake blood pressure (average of 2 hours of readings before wake-up) |
| Rising surge | Blood pressure on rising (single reading after wake-up) minus blood pressure before wake-up (single reading before wake-up) |
| Morning nighttime difference | Two morning blood pressure readings (after 7 am) minus the average nighttime blood pressure |
| Morning blood pressure | Average morning blood pressure for 2 hours after wake-up |
| Morning evening difference | Morning blood pressure (average of self-monitored blood pressure readings taken in the morning) minus evening blood pressure (average of self-monitored blood pressure readings taken in the evening) |
| Morning blood pressure power | The product of the rate of the rise (change over time) and the amplitude (day–night difference) of morning blood pressure |
Table 4.
Definition, threshold, and method measurement of morning blood pressure surge examined in included studies
| Study | Definition of MBPS | Categorical or continuous variable? | Threshold for pathological MBPS | How was the threshold defined? | Method of BP measurement | Type of monitor(s) used | Definition of wake-up? | CVD endpoint studied |
|---|---|---|---|---|---|---|---|---|
| Amici et al. 5 | Sleep-trough surge | Categorical | >34mm Hg | Top decile of MBPS | 24-h ABPM | TM-2430 | Patient diary | CVD events |
| Amodeo et al. 26 | Sleep-trough surge | Categorical | >41mm Hg | Not stated | 24-h ABPM | Not specified | Not stated | CVD events |
| Dolan et al. 6 | Prewaking surge | Continuous | Not stated | Not stated | 24-h ABPM | Not specified | Not stated | CVD events and mortality |
| Gosse et al. 19 | Rising surge | Categorical | >153mm Hg | Quartiles of MBPS | 24-h ABPM | Spacelabs 5200 or Diassys 200 | Not stateda | CVD events |
| Gosse et al. 11 | Rising surge | Categorical | 4th quartile of MBPS | Quartiles of MBPS | 24-h ABPM | Spacelabs 5200, Diassys 200/Integra | Not stateda | CVD events and mortality |
| Hermida et al. 27 | Sleep-trough surge | Continuous | None used | Not stated | 24-h ABPM | Not stated | Not stated | CVD riskb |
| Iqbal et al. 22 | Morning–nighttime difference | Categorical | >20mm Hg | Not stated | 24-h ABPM | Not stated | Not stated | CVD events and mortality |
| Israel et al. 10 | Sleep-trough surge | Continuous | None used | Not stated | 24-h ABPM | Not specified | Patient diary | All-cause mortality |
| Israel et al. 10 | Prewaking surge | Continuous | None used | Not stated | 24-h ABPM | Not specified | Patient diary | All-cause mortality |
| Israel et al. 10 | Rising surge | Both | >12mm Hg | Median MBPS | 24-h ABPM | Not specified | Patient diary | All-cause mortality |
| Kario et al. 7 | Sleep-trough surge | Both | >55mm Hg | Top decile of MBPS | 24-h ABPM | TM-2425/2421 or ABPM-630 | Not stated | Stroke events |
| Kario et al. 7 | Prewaking surge | Continuous | >55mm Hg | Top decile of MBPS | 24-h ABPM | TM-2425/2421 or ABPM-630 | Not stated | Stroke events |
| Kario et al. 23 | Not stated | Not stated | Not stated | Not stated | 24-h ABPM | Not stated | Not stated | Stroke events |
| Li et al. 4 | Sleep-trough surge | Categorical | >37mm Hg | Top decile of MBPS | 24-h ABPM | Spacelabs 90202/90207; TM-2421; ABPM-630 | Patient diary | CVD events and mortality |
| Li et al. 4 | Prewaking surge | Categorical | >28mm Hg | Top decile of MBPS | 24-h ABPM | Spacelabs 90202/90207; TM-2421; ABPM-630 | Patient diary | CVD events and mortality |
| Metoki et al. 8 | Prewaking surge | Both | >25mm Hg | Quintiles of MBPS | 24-h ABPM | ABPM-630 | Patient diary | Stroke events |
| Metoki et al. 8 | Sleep-trough surge | Both | >40mm Hg | Quintiles of MBPS | 24-h ABPM | ABPM-630 | Patient diary | Stroke events |
| Metoki et al. 24 | Morning BP | Continuous | None used | Not stated | 24-h ABPM | ABPM-630 | Patient diary | Stroke events |
| Nishinaga et al. 21 | Morning evening difference | Categorical | >15mm Hg | Not stated | Home BP | Omron HEM-755C | Not stated | CVD events and mortality |
| Reid et al. 25 | Morning BP power | Categorical | Not stated | Not stated | 24-h ABPM | Not specified | Not stated | All-cause mortality |
| Verdecchia et al. 9 | Sleep-trough surge | Categorical | >36mm Hg | Top quartile of MBPS | 24-h ABPM | Spacelabs 5200/90202/90207 | Patient diary | CVD events and mortality |
| Verdecchia et al. 9 | Prewaking surge | Categorical | >27.5mm Hg | Top quartile of MBPS | 24-h ABPM | Spacelabs 5200/90202/90207 | Patient diary | CVD events and mortality |
| Yano et al. 20 | Rising surge | Categorical | Not stated | Quartiles of MBPS | 24-h ABPM | Not specified | Not stated | Stroke events |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; CVD, cardiovascular disease; MBPS, morning blood pressure surge.
aPatients manually triggered the blood pressure monitor to take a reading upon rising.
bSpecific endpoints not defined.
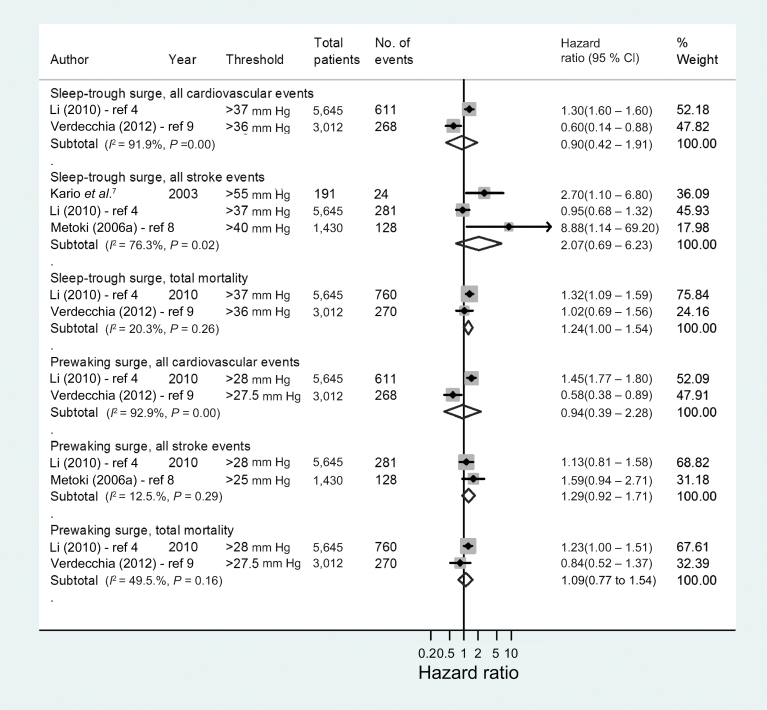
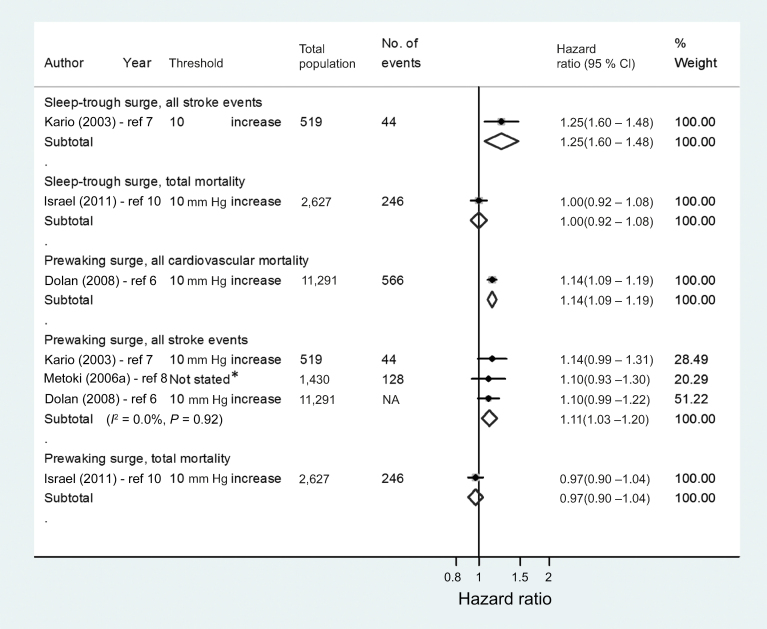
Because of the low number of studies eligible for the pooled analyses, it was not possible to compare all definitions or thresholds of MBPS or carry out subgroup analyses by methodological quality. We focused our pooled analyses on studies examining comparable definitions of MBPS. The 2 most commonly used definitions of MBPS (sleep-trough surge and prewaking surge) were therefore pooled in separate meta-analyses grouped by outcome variable (Figures 3 and 4). There was no evidence of an association between the MBPS, defined by a predetermined threshold, and all cardiovascular events, stroke events, or all-cause mortality (Figure 3). However, when the MBPS was analyzed as a continuous variable, a 10 mm Hg increase in the prewaking surge was associated with an increased risk of all stroke events (n = 3 studies; HR = 1.11, 95% CI = 1.03–1.20) (Figure 4). Metoki et al.,8 which was included in this result, failed to report the unit of increase in MBPS associated with stroke events, although other analyses reported in this article examined an increase of 13.8mm Hg (1 SD). Even with the removal this study, the association between increasing prewaking surge and stroke remained (n = 2 studies; HR = 1.11, 95% CI = 1.02–1.21). Only 1 study related the sleep-trough surge to stroke events on a continuous scale, and this was also associated with an increased risk (HR = 1.25, 95% CI = 1.06–1.48).7
Figure 3.
Forest plot of adjusted hazard ratios (HRs) depicting the risk of cardiovascular morbidity and/or mortality with an exaggerated morning blood pressure surge. Data were analyzed as categorical variables (using a threshold value to define an exaggerated morning blood pressure surge).
Figure 4.
Forest plot of adjusted hazard ratios depicting the risk of cardiovascular morbidity and/or mortality with an exaggerated morning blood pressure surge. Data were analyzed as continuous variables and presented here per 10 mm Hg increase in morning blood pressure surge. *Unit of increase relating to this hazard ratio was not reported. Other hazard ratios reported in this article referred to a single standard deviation increase in prewaking surge equivalent to 13.8 mm Hg.8 Abbreviation: NA, not available.
All included studies were adjusted for confounding, but potential sources of bias varied between studies (Supplementary Table S2). All studies adjusted for age and mean systolic blood pressure, and all but 1 adjusted for sex,7 but only 3 of 6 studies corrected for dipping status.4,7,9 The heterogeneity between studies was considerable in those examining the association between the sleep-trough surge or prewaking surge and cardiovascular events (I 2 = 91.9%–92.9%; P < 0.001).4,9 However, in studies investigating the association between the prewaking surge (analyzed as a continuous variable) and all stroke events, heterogeneity was low (I 2 = 0.0%; P = 0.92).6–8
DISCUSSION
This study systematically reviewed all existing literature evaluating the association between MBPS and subsequent cardiovascular disease. No clear evidence of an association between MBPS and all cardiovascular disease or stroke events or all-cause mortality was found when the surge was defined by a predetermined threshold, confirming the findings of recent prospective studies.9,10 However, using a continuous variable to describe the morning surge, there was evidence of an association with all stroke events in patients with hypertension: for every 10 mm Hg increase in (prewaking) MBPS, the risk of stroke also increased by 11%. This suggests that the relationship between MBPS and outcome is more complex than can be identified simply using a single threshold and is perhaps unsurprising given that analysis of candidate continuous predictors on their original scale has more power and is less prone to bias than dichotomization.28,29 However, given the paucity and quality of studies examining the MBPS in this way, further work is needed, perhaps through reanalysis of existing data, before definitive recommendations for clinical practice can be made.
This study used a thorough and extensive search strategy in a large number of research literature databases to capture existing prospective studies relating MBPS to cardiovascular disease endpoints. Despite screening a large number of potentially relevant studies (n = 2,964), only 17 articles fulfilled the study inclusion criteria, and only 6 of these could be pooled in a meta-analysis. This limited the extent to which different definitions and thresholds of MBPS could be compared as originally planned. This was particularly evident in the assessment of the MBPS as a continuous variable, where only the association between the prewaking surge and all stroke events was examined by >1 study and the 2 largest, highest quality studies4,9 could not be included.
Not all of the studies included in our pooled analyses were directly comparable. Most dichotomized the sample population by a particular threshold level of MBPS and compared those with an exaggerated MBPS against the rest of the population. The choice of threshold often differed across studies, as would be expected given that a pathological MBPS differs by various factors such as hypertensive status, age, and ethnicity.2 These meta-analysis results relate to the association at some average threshold value, which may go some way to explaining why the association between exaggerated MBPS and cardiovascular disease was not significant when data were examined in this way.
The study by Verdecchia et al. 9 divided the sample population into quartiles by level of MBPS and individually compared patients with an exaggerated MBPS against those from each of the 3 other quartiles of MBPS level. In our pooled analyses, HRs comparing those with an exaggerated MBPS against those with a minimal MBPS (lowest quartile of MBPS) were used. Thus the estimates of association between MBPS and cardiovascular endpoints from this study are likely to be more pronounced compared with that seen in other studies. It should also be noted that there were differences in the adjustment for other prognostic factors (confounders) used across studies (Supplementary Table S2). Despite this, it is a strength that studies adjusted for multiple variables, thus allowing the independent prognostic association for MBPS and outcome to be summarized.
One study included in our pooled analyses was that of Li et al.,4 which examined data from the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome.30 This database includes patients from studies conducted around the world, including those from the Ohasama Study31 and the Allied Irish Bank study.32 It is possible that these same patients may have been included in other studies identified by this review,6,8 although it was not possible to confirm this from the data available. This potential overlap only affected analyses of the association between sleep-trough prewaking surge and stroke events (examined using a threshold to define exaggerated MBPS) (Figure 3), neither of which showed significant results, and thus the impact on the overall findings of this study are likely to be minimal.
Our study did not explicitly set out to consider the influence of nocturnal dipping status on cardiovascular disease risk, although some studies included in the meta-analysis did adjust their findings for dipping status in the sample population (Supplementary Table S2).4,6,7 A lack of nocturnal dip is considered to be a significant independent risk factor for cardiovascular disease, despite such patients having only a small MBPS. This apparent contradiction may explain some of the inconsistences in association between MBPS and cardiovascular disease observed here. This review was not designed to compare the associations of MBPS and nocturnal dipping status with cardiovascular disease, but future work should consider these associations together, rather than in isolation.
The exaggerated MBPS was originally proposed as a prognostic factor for stroke in 2003.7 In a group of 519 elderly hypertensive patients, it was shown that a 10 mm Hg increase in MBPS resulted in a 25% increased risk of clinical stroke events, and the authors proposed a sleep-trough MBPS of >55mm Hg as pathological. A subsequent review,2 published in 2010, summarized the existing literature relating specific thresholds of MBPS to cardiovascular endpoints and concluded that it was an important risk factor. Since then, more recent studies have shown contradictory findings,9,10 and the inclusion of these and others19,21,23–27 in our review has resulted in subtly different conclusions: namely, that although there was no significant association between MBPS above a predetermined threshold and increased risk of cardiovascular disease, there was evidence of a relationship between increasing levels of MBPS (analyzed on a continuous scale) and increased risk of stroke events in hypertensive patients. This finding is perhaps not surprising given that analysis of candidate continuous predictors on their original scale has more power to detect associations with a given outcome variable.28,29
This issue is also pertinent in the diagnosis of hypertension, where for many years, high blood pressure has been defined as blood pressure above a specific threshold,33 despite the linear relationship between cardiovascular disease risk and increasing blood pressure.34 The appropriate threshold for treatment of hypertension has long been debated35 without worldwide consensus.14,36,37 Indeed, some have suggested that thresholds should be abandoned in favor of a risk-based approach,38 and this has been adopted in Australia36 and New Zealand.37
MBPS is an important concept in clinical practice, not least because it has been proposed as a cause of wake-up stroke,2,3 which is not amenable to treatment with thrombolysis because of lack of knowledge of onset time.39 Identifying MBPS is now realistic with the increased uptake of ambulatory blood pressure monitoring in routine clinical practice.14 This study found some evidence that an increasing MBPS is associated with an increased stroke risk, and conceivably this could allow inclusion in risk calculation tools. However, because of the limited number of studies, this finding requires further investigation. Indeed, of the 3 studies that analyzed the data in this way, 1 was only published as a conference proceeding and the remaining 2 studies were conducted in Japanese populations where the risk of stroke is high; thus the generalizability of these findings is unclear. Further work could involve reanalysis of existing patient data from previous studies in an individual patient data meta-analysis.40 Should future studies confirm an increasing MBPS as a prognostic factor for cardiovascular disease, more thought will be required to establish how such a marker can be used effectively (i.e., at what point should treatment regimens be adjusted to account for increasing MBPS) given that for diagnosis and treatment decisions, markers using predetermined thresholds are easier to implement in routine clinical practice.
This study found some evidence that increasing levels of MBPS are associated with increased risk of stroke. This was only the case when the MBPS was measured and analyzed as a continuous variable, perhaps because of the increased power to detect associations with the specified outcome variable. Further studies examining MBPS in this way are needed to accurately define this relationship to inform routine clinical practice.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank David Moore for his advice in the design of this work and Jeremy Nagle and Paul Terry at the British Library for their assistance in sourcing the full-text articles screened for inclusion in this work. This work is independent research supported by the National Institute for Health Research School for Primary Care Research (project No. 130). J.P.S. was supported by the National Institute for Health Research (NIHR) Birmingham and Black Country Collaboration for Leadership in Applied Health Research and Care and now holds a Medical Research Council Strategic Skills Postdoctoral Fellowship. R.J.M. holds an NIHR Professorship. The views and opinions expressed are those of the authors and do not necessarily reflect those of the NHS, NIHR, or the Department of Health. This study was registered on the PROSPERO International prospective register of systematic reviews (registration No. CRD42012002091).
REFERENCES
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin AA, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De LD, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kario K. Morning surge in blood pressure and cardiovascular risk evidence and perspectives. Hypertension 2010; 56:765–773. [DOI] [PubMed] [Google Scholar]
- 3. Kario K, White WB. Early morning hypertension: what does it contribute to overall cardiovascular risk assessment? J Am Soc Hypertens 2008; 2:397–402. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp-Pedersen C, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Sandoya E, Kawecka-Jaszcz K, Ibsen H, Imai Y, Wang J, Staessen JA. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010; 55:1040–1048. [DOI] [PubMed] [Google Scholar]
- 5. Amici A, Cicconetti P, Sagrafoli C, Baratta A, Passador P, Pecci T, Tassan G, Verrusio W, Marigliano V, Cacciafesta M. Exaggerated morning blood pressure surge and cardiovascular events. A 5-year longitudinal study in normotensive and well-controlled hypertensive elderly. Arch Gerontol Geriatr 2009; 49:e105–e109. [DOI] [PubMed] [Google Scholar]
- 6. Dolan E, McCormack P, Staessen JA, O’Brien E. The morning surge in systolic blood pressure predicts cardiovascular mortality: dublin outcome study. J Hypertens 2008; 26:S30. [Google Scholar]
- 7. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kurota T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives - A prospective study. Circulation 2003; 107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 8. Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline—the Ohasama study. Hypertension 2006; 47:149–154. [DOI] [PubMed] [Google Scholar]
- 9. Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension 2012; 60:34–42. [DOI] [PubMed] [Google Scholar]
- 10. Israel S, Israel A, Ben-Dov IZ, Bursztyn M. The morning blood pressure surge and all-cause mortality in patients referred for ambulatory blood pressure monitoring. Am J Hypertens 2011; 24:796–801. [DOI] [PubMed] [Google Scholar]
- 11. Gosse P, Lasserre R, Minifie C, Lemetayer P, Clementy J. Blood pressure surge on rising. J Hypertens 2004; 22:1113–1118. [DOI] [PubMed] [Google Scholar]
- 12. Stergiou GS, Mastorantonakis SE, Roussias LG. Morning blood pressure surge: the reliability of different definitions. Hypertens Res 2008; 31:1589–1594. [DOI] [PubMed] [Google Scholar]
- 13. Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FR, Hodgkinson J, Mant J, Martin U, Williams B, Wonderling D, McManus RJ. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011; 378:1219–1230. [DOI] [PubMed] [Google Scholar]
- 14. National Institute for Health and Care Excellence. Hypertension: clinical management of primary hypertension in adults. http://www.nice.org.uk/CG127 Accessed 1 May 2014.
- 15. Altman DG. Practical Statistics for Medical Research. 1st ed. Chapman and Hall/CRC: Boca Raton, FL, 1991. [Google Scholar]
- 16. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 17. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006; 144:427–437. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 19. Gosse P, Cipriano C, Bemurat L, Mas D, Lemetayer P, N’Tela G, Clementy J. Prognostic significance of blood pressure measured on rising. J Hum Hypertens 2001; 15:413–417. [DOI] [PubMed] [Google Scholar]
- 20. Yano Y, Hoshide S, Shimada K, Kario K. Additional impact of morning haemostatic factors and blood pressure surge on stroke in older Japanese hypertensives. J Clin Hypertens 2011; 13(Suppl. 1): A62. [DOI] [PubMed] [Google Scholar]
- 21. Nishinaga M, Takata J, Okumiya K, Matsubayashi K, Ozawa T, Doi Y. High morning home blood pressure is associated with a loss of functional independence in the community-dwelling elderly aged 75 years or older. Hypertens Res 2005; 28:657–663. [DOI] [PubMed] [Google Scholar]
- 22. Iqbal P, Stevenson L. Cardiovascular outcomes in patients with normal and abnormal 24-hour ambulatory blood pressure monitoring. Int J Hypertens 2010; 2011:786912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kario K, Shimada K, Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens (New York) 2004; 26:177–189. [DOI] [PubMed] [Google Scholar]
- 24. Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hara A, Hirose T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance of night-time, early morning, and daytime blood pressures on the risk of cerebrovascular and cardiovascular mortality: the Ohasama Study. J Hypertens 2006; 24:1841–1848. [DOI] [PubMed] [Google Scholar]
- 25. Reid C, Head G, Lukoshkova E, Shiel L, Owen A, Wing L. The power of the morning blood pressure surge and its relation to long-term survival in the 2nd Australian National Blood Pressure Study (ANBP2). J Hypertens 2010; 28:e15. [Google Scholar]
- 26. Amodeo C, Picotto JC, Cordeiro AC, dos Santos CC, Guimaraes GG, Bezerra Fonseca KDM, Martins RF. Early morning hypertension (EMH) and cardiovascular risk. Circulation 2012; 125:e876. [Google Scholar]
- 27. Hermida RC, Ayala DE, Mojon A, Fernandez JR. A greater morning blood pressure surge is associated with lower, not higher, cardiovascular risk. J Clin Hypertens 2012; 14:18. [Google Scholar]
- 28. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86:829–835. [DOI] [PubMed] [Google Scholar]
- 29. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006; 25:127–141. [DOI] [PubMed] [Google Scholar]
- 30. Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Li Y, Dolan E, Tikhonoff V, Seidlerova J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang J, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, O’Brien E. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit 2007; 12:255–262. [DOI] [PubMed] [Google Scholar]
- 31. Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 32. O’Brien E, Murphy J, Tyndall A, Atkins N, Mee F, McCarthy G, Staessen J, Cox J, O’Malley K. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens 1991; 9:355–360. [DOI] [PubMed] [Google Scholar]
- 33. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 1993; 153:154–183. [PubMed] [Google Scholar]
- 34. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 35. Ramsay LE, ul Haq I, Yeo WW, Jackson PR. Interpretation of prospective trials in hypertension: do treatment guidelines accurately reflect current evidence? J Hypertens 1996; 14:S187–S194. [PubMed] [Google Scholar]
- 36. National Heart Foundation of Australia (National Blood Pressure and Vascular Disease Advisory Committee). Guide to the management of hypertension 2008: assessing and managing raised blood pressure in adults. http://www.heartfoundation.org.au Accessed 4 March 2014.
- 37. New Zealand Guidelines Group. The assessment and mangement of cardiovascular risk. http://www.nzgg.org.nz Accessed 4 March 2014.
- 38. Weir MR. Risk-based classification of hypertension and the role of combination therapy. J Clin Hypertens (Greenwich) 2008; 10:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wahlgren N, Ahmed N, Davalos A, Hacke W, Millan M, Muir K, Roine RO, Toni D, Lees KR. Thrombolysis with alteplase 3–4.5h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372(9646):1303–1309. [DOI] [PubMed] [Google Scholar]
- 40. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340:c221. [DOI] [PubMed] [Google Scholar]
- 41. Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Itoh O, Bando T, Sakuma M, Fukao A, Satoh H, Hisamichi S, Abe K. Prediction of mortality by ambulatory blood pressure monitoring versus screening blood pressure measurements: a pilot study in Ohasama. J Hypertens 1997; 15:357–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.