Abstract
Little is known about the digestive process in infants. In particular, the chronological activity of enzymes across the course of digestion in the infant remains largely unknown. To create a temporal picture of how milk proteins are digested, enzyme activity was compared between intact human milk samples from three mothers and the gastric samples from each of their 4–12 day postpartum infants, 2 h after breast milk ingestion. The activities of 7 distinct enzymes are predicted in the infant stomach based on their observed cleavage pattern in peptidomics data. We found that the same patterns of cleavage were evident in both intact human milk and gastric milk samples, demonstrating that the enzyme activities that begin in milk persist in the infant stomach. However, the extent of enzyme activity is found to vary greatly between the intact milk and gastric samples. Overall, we observe that milk-specific proteins are cleaved at higher levels in the stomach compared to human milk. Notably, the enzymes we predict here only explain 78% of the cleavages uniquely observed in the gastric samples, highlighting that further investigation of the specific enzyme activities associated with digestion in infants is warranted.
Keywords: milk enzymes, enzyme activity, digestive enzymes, infant digestion, proteolytic enzymes, human milk, indigenous enzymes
Introduction
Human milk delivers essential proteins, lipids, sugars, and minerals to the developing neonate. Additionally, human milk is known to contain a variety of indigenous enzymes. Recently, we employed mass spectrometry-based peptidomics to show that milk from healthy mothers contains hundreds of endogenous peptides.1 These peptides were analyzed for enzymatic cleavage patterns, which demonstrated the activity of a range of indigenous proteases in human milk.2
For the most part, the peptide degradation products and cleavage agents of the human infant stomach remain largely unstudied. However, there has been considerable characterization of the principal gastric protease, pepsin. Pepsin, which is known to be present in the stomach of infants by 16 weeks of gestation,3 functions best at an acidic pH and is irreversibly denatured at pH 7.4 For at least 13 days postpartum, term infants have a gastric pH of 5–7 for up to an hour postingestion.5 Accordingly, this high pH range, due to low acid production by infant parietal cells, combined with the high buffering capacity of milk,6 has been interpreted to imply that little to no pepsin-induced proteolysis occurs in the infant stomach.7
To compensate for variability in the maturation of the neonatal gastrointestinal tract, it has been suggested that indigenous milk enzymes might fill developmental gaps in enzymatic digestion. For example, milk enzymes digest milk proteins to release nutrients and functional peptides that would otherwise be unavailable to the newborn.8,9 Indeed, the stage of lactation is known to affect the enzyme concentration in human milk, with the highest concentration of enzymes being found soon after birth.10
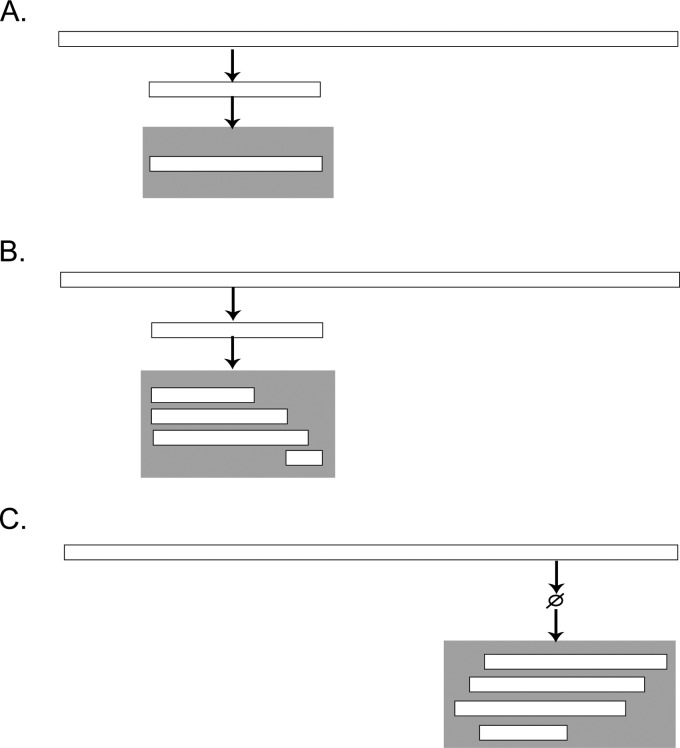
To further our understanding of the activity of indigenous human milk enzymes, we compared the protein cleavage patterns of human milk at two significant time-points: prior to ingestion (as sampled from the mother’s breast) and 2 h postingestion (as sampled from the infant’s stomach). Comparing the cleavage patterns at these two time points allows us to determine which, if any, native human milk enzyme activities are preferentially retained in the infant stomach and which are not. Upon reaching the infant stomach, one of three possible outcomes for human milk proteins and peptides (resultant from native enzyme cleavage) can be expected: (1) they reach the infant stomach unchanged; (2) they are subject to further cleavage by indigenous milk enzymes; (3) novel cleavage patterns from gastric enzymes emerge (see Figure 1).
Figure 1.
The possible scenarios that can be expected when comparing the peptides in intact human milk with the peptides found in the infant stomach. In each panel, the parent milk protein is represented by the top horizontal bar. The peptide in breast milk is represented by the smaller white bar, and the gray box represents when a peptide is in the stomach. In (A), the peptides present in intact human milk are found in the stomach and undergo no gastric digestion but can multiply in copy number, whereas in (B), peptides from milk are subject to further digestion in the stomach leading to smaller fragments. Finally, (C) represents peptides that are only achieved by digestion of gastric enzymes (i.e., they are not present in intact milk).
Here, we show that several of the enzymes present in human milk continue their activity in the infant stomach, and in many cases display increased activity. While the observed increase in specific cleavage patterns in the stomach can be explained in part by known gastric enzymes, other increased cleavage patterns are less expected, as they derive from enzymes uncharacteristic of the stomach.
Materials and Methods
This study uses the peptidomics data from Dallas et al.11 of milk samples from 3 human mothers and the gastric aspirates from their postpartum infants. The experimental approach, including sample extraction, mass spectrometry, database searching, and relative abundance extraction, is described fully in Dallas et al.11 The sample collection and methods are described briefly below.
Sample Collection
Following informed consent, intact milk samples were obtained from healthy mothers who delivered at term. The term infants were hospitalized due to health problems unrelated to the gastrointestinal tract. The infants’ conditions precluded normal feeding; therefore, a naso-gastric tube was placed for each. The infants were fed mother’s expressed breast milk with no fortification. The milk samples were expressed exclusively by breast pump into a sterile plastic container, taking 10–15 min (see Dallas et al.11 for more details). The milk samples were immediately stored in home freezers and later transported on ice to the Neonatal Intensive Care Unit of the UC Davis Children’s Hospital. The infants were fed the expressed milk via the naso-gastric tubes over 30 min. Two hours after the initiation of the feeding, a fraction of the gastric contents of each infant was collected back through the tube via suction. The intact milk and gastric samples were stored at −40 °C and then transported to the UC Davis laboratory on dry ice and stored at −80 °C.
Sample Preparation
A 100 μL sample of mother’s expressed milk and 100 μL of infant’s gastric fluid were delipidated12 to remove the cream layer from the samples. Proteins in the infranate samples were precipitated by trichloroacetic acid.13 The supernatant was removed and applied to a 96-well C18 solid-phase extraction plate to purify peptides. Peptides were eluted in 80% acetonitrile (ACN), 0.1% trifluoroacetic acid (TFA). Eluted peptides were dried and rehydrated in nanopure water prior to quadrupole time-of-flight (Q-TOF) analysis.
Peptide Analysis
The analysis was performed with the Agilent nanoliquid chromatography chip-cube 6520 quadrupole time-of-flight tandem mass spectrometer. The chip employed contained an enrichment and analytical column packed with a C18 stationary phase. Each sample was run in triplicate on the Q-TOF in mass spectrometry (MS) only mode. Instrument parameters were as per Dallas et al.11
Peptide Identification, Library Creation, and Peak Area Extraction
Peptides were analyzed in MS/MS mode and analyzed in X!Tandem14 against a library of human milk proteins derived from previous human milk proteomes15−17 (parameters described in Dallas et al.11). The results from X!Tandem for all samples were compiled into a peptide library that was applied to extract peptide peak areas from each sample.
Consensus Sample Creation
From the mass spectrometry runs of the three human milk samples, a single representative consensus peptide profile was created. The consensus sample was achieved by retaining only peptides that were detected in a minimum of 2 samples. The same procedure was equivalently carried out for the gastric samples.
Enzymatic Prediction in Intact Milk
To predict which enzymes are active in intact human milk based on the MS-identified peptide sequences, an online software application, EnzymePredictor, was employed.18 EnzymePredictor, designed to examine cleavage patterns and predict the causative enzymes of peptide profiles, contains the cleavage specificities of 35 enzymes that are well documented in the literature. These enzymes are statistically compared to identify the most likely patterns and enzymes that explain the observed data.18 Here, we only considered human enzymes to present a better account of indigenous enzyme activity. Cleavages at the N-terminus, representing the removal of the signal peptide from the milk protein sequence, were assigned to unknown and thus have not influenced our analyses. A first run was performed to identify all possible enzymes or chemical cleavage agents. The peptides were then run against a list of enzymes determined in previous work.18 If an enzyme or cleavage agent was not of human origin and its specificity overlapped 100% with other predicted enzymes, then it was removed from our predictions. Removed cleavage agents included formic acid and cyanobromide. Removed bacterial enzymes included Lys-C, clostripain, Asp-N endopeptidase, Glutamyl endopeptidase and Staphylococcal peptidase. If an enzyme cleavage pattern was similar to that of another enzyme, the enzyme with the broader cleavage specificity was selected. Enzymes with similar activities were chymotrypsin low affinity and chymotrypsin high affinity (differing by only a single possible amino acid cleavage site), and pepsin acting at pH = 1.3 and pH > 2 (differing by 2 potential amino acid cleavage sites).
Enzymatic Prediction in Gastric Aspirates
To determine gastric enzyme activity patterns, EnzymePredictor was also applied to the MS-derived peptides of the gastric aspirates. Similarly to the analysis of the intact human milk samples, a first run was performed to identify all possible enzymes.18 Enzymes were removed from the predictions if they met the criteria outlined above for the intact human milk samples. Removed cleavage agents and enzymes included formic acid, cyanobromide, iodosobenzoic acid, Lys-C, clostripain, Asp-N endopeptidase, Glutamyl endopeptidase and Staphylococcal peptidase. As with the intact milk samples, if an enzyme’s cleavage specificity was encompassed by the specificity of another, the enzyme with the broader specificity was retained in our predicted list. Again, this was true for chymotrypsin low affinity and high affinity, and pepsin acting at pH 1.3 and pH > 2.
Peptide Abundance Analysis
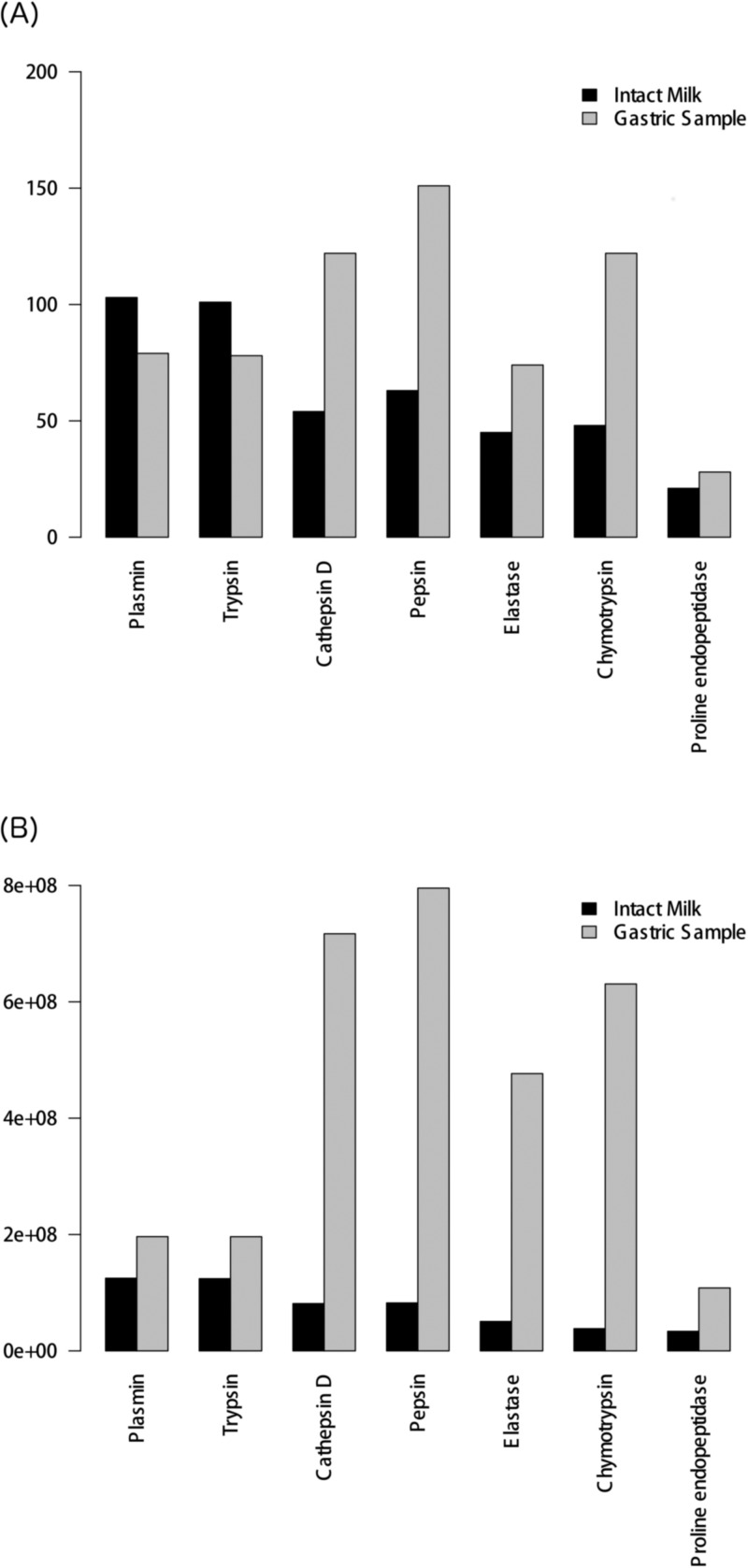
To quantify the activity of an enzyme, two approaches were used. In the first approach, the number of cleavages performed on each peptide was considered (Figure 2A). In the second approach, the abundances of the different peptides in terms of ion counts were employed (Figure 2B). While both of these methods have individual advantages and disadvantages, they converge on a similar trend for the overall activity of enzymes in milk (intact milk versus gastric sample; Figure 2).
Figure 2.
Comparison between the activity of enzymes in intact milk and in the infant gastric aspirates. (A) Bar plot of the total number of cleavages in intact milk and in the infant gastric aspirates. Enzymes taking part in protein digestion are represented on the x-axis, with the total number of cleavages occurring at peptide termini found on the y-axis. (B) Bar plot of number of unique peptides associated with the action of an enzyme multiplied by the corresponding peptide abundance (ion counts). Enzymes taking part in protein digestion are represented on the x-axis, with the sum of peptide abundance found on the y-axis.
Differences in Enzyme Activity between Intact Milk and Gastric Aspirates
We calculated change in enzyme activity between intact human milk and the gastric aspirates as follows: for each enzyme we subtracted the number of unique peptides attributed to that enzyme (N- or C-terminal cleavage) in intact milk from the number of unique peptides attributed to the same enzyme in gastric milk (Table 3). This analysis was extended to consider the total number of all peptides (including the various modification states of each peptide sequence) released by an enzyme, thus including any duplicates of the same peptide sequence. In analyzing the various modification states, our observations were the same as when modification states were not considered and each peptide sequence was included only once (data not shown).
Table 3. Comparison of the Cleavages in Intact Milk versus Gastric Milk for Each of the 7 Predicted Enzymes.
| enzymes | total number of cleavages in human intact milk | total number of cleavages in human gastric aspirates | gastric specific cleavage | fold increase (−) in intact milk (+) in gastric aspirates |
|---|---|---|---|---|
| Plasmin | 103 | 79 | 9 | –1.3 |
| Trypsin | 101 | 78 | 9 | –1.3 |
| Cathepsin D | 54 | 122 | 58 | +2.3 |
| Pepsin | 63 | 151 | 78 | +2.4 |
| Elastase | 45 | 74 | 27 | +1.6 |
| Chymotrypsin | 48 | 122 | 60 | +2.5 |
| Proline endopeptidase | 21 | 28 | 9 | +1.3 |
Results and Discussion
Enzyme Activity in Human Milk
In previous work, we analyzed milk from two healthy mothers to determine the range of enzymes involved in the proteolytic cleavage of human milk proteins.2 Here, we replicate this search on samples from three mothers and extend it to include corresponding gastric samples from their infants in order to chronologically investigate the digestion of human milk proteins.
Mass spectrometry (MS) was applied to three human milk samples to determine the peptide sequences present. When the cleavage patterns present in the MS-peptide profile were examined, 7 enzymes were identified as active in human milk samples (illustrated in Table 1). In line with our previous work,2 we find a considerable number of peptide fragments in the human milk samples that are consistent with the cleavage of milk proteins by indigenous enzymes. The highest amount of cleavage occurs after K and R residues (P1 position), a pattern which is characteristic of plasmin or trypsin cleavage (Table 1; Figure 2A). Additionally, considerable cleavage is present after F, L, W or Y (P1 position), consistent with the cleavage of pepsin- or chymotrypsin-like enzymes. Cleavages after A, I, L, P, and V residues were also present, in line with the cleavage potential of the enzymes cathepsin D or elastase. Other, lower yield, cleavage patterns observed at the termini of peptides are consistent with the action of proline endopeptidase (Table 1; Figure 2A).
Table 1. Details of the Enzymes That Take Part in the Digestion of the Human Milk Proteinsa.
| enzymes | number of cleavages at the N-terminus of the milk peptides | number of cleavages at the C-terminus of the milk peptides | total number of cleavages of the milk peptides |
|---|---|---|---|
| Plasmin | 65 | 38 | 103 |
| Trypsin | 63 | 38 | 101 |
| Cathepsin D | 31 | 23 | 54 |
| Pepsin 1 | 19 | 29 | 48 |
| Elastase | 23 | 22 | 45 |
| Chymotrypsin low 1 | 27 | 14 | 41 |
| Pepsin 2* | 18 | 9 | 27 |
| Proline endopeptidase | 7 | 14 | 21 |
| Chymotrypsin low 4* | 2 | 5 | 7 |
Enzymes containing an asterisk indicate the same enzyme but having two slightly different cleavage patterns.
It must be noted that some of the amino acid preferences detected to either side of cleavage sites in human milk match those seen experimentally when bovine caseins are digested by cathepsin B19 and G;20 enzymes that are likely to be indigenous to bovine milk (see Kelly et al.21). However, experimental evidence indicates that the cleavage specificity of human cathepsin G overlaps with that of both chymotrypsin and trypsin,22 making it difficult to separate the individual contributions of these enzymes. The specificity of human cathepsin B and G against a diverse range of proteins has not been sufficiently characterized to date, therefore they are not featured in this current EnzymePredictor analysis.
Here, like in our previous work,2 we identify pepsin-like activity in human milk. While the amino acid cleavage sites of pepsin overlap with some of chymotrypsin, we find considerable cleavage that is uniquely associated with pepsin (see Figure 2A). The detection of a pepsin-like enzyme is surprising given the high pH of milk compared to the acidic pH necessary for optimal pepsin activity. This finding may be explained in two different ways: (1) pepsin may exist in human milk, similarly to other indigenous enzymes such as plasmin, but has low activity because of the high pH of milk; (2) the observed cleavages could be created by an enzyme that has a pepsin-like pattern, which is not included in our current list of enzyme patterns, see Vijayakumar et al.,18 and is not yet known to be present in human milk. While in previous work2 we do find some evidence that low levels of pepsin are expressed in the mammary gland, elucidating which explanation is more likely remains to be determined.
Enzyme Activity in Gastric-Digested Human Milk
Two-hour postprandial gastric samples were collected from three term infants aged 4–12 days, and the released peptide fragments were identified by mass spectrometry. The peptide sequences were subsequently analyzed using EnzymePredictor18 to determine the cleavage patterns present and their associated enzymes. The 7 predicted enzymes (shown in Table 2) are identical to those predicted for intact human milk (see Table 1).
Table 2. Details of the Enzymes That Take Part in the Digestion of Proteins inside the Infant’s Stomacha.
| enzymes | number of cleavages at the N-terminus of the milk peptides | number of cleavages at the C-terminus of the milk peptides | total number of cleavages of the milk peptides |
|---|---|---|---|
| Cathepsin D | 70 | 52 | 122 |
| Chymotrypsin low 1 | 58 | 54 | 112 |
| Pepsin 2 | 52 | 47 | 99 |
| Pepsin 1* | 46 | 51 | 81 |
| Plasmin | 51 | 28 | 79 |
| Trypsin | 50 | 28 | 78 |
| Elastase | 41 | 33 | 74 |
| Proline endopeptidase | 11 | 17 | 28 |
| Chymotrypsin low 4 | 2 | 3 | 5 |
| Chymotrypsin low 3 | 3 | 2 | 5 |
| Chymotrypsin low 2 | 1 | 0 | 1 |
Enzyme containing an asterisk indicates the same enzyme but having two slightly different cleavage patterns.
In this postingestion sample, enrichment in cleavage after F, L, W, and Y (P1 position) is present, consistent with pepsin- or chymotrypsin-like activity (Table 2; Figure 2A). There are 89 cleavages detected in the gastric sample that can equally be accounted for by either chymotrypsin- or pepsin-like activity. However, a further 33 observed cleavages are found to be uniquely chymotrypic, and 62 uniquely peptic, suggesting that it is unlikely that the predicted chymotrypsin activity is due to an overlap in specificity with pepsin. Rather, the data suggest that both chymotrypsin-like and pepsin-like enzymes are active in the infant stomach.
Enrichment in cleavage after A, I, L, P, and V is also seen, in a pattern that is in accordance with the activity of cathepsin D or elastase (Table 2; Figure 2A). The frequency of cleavage after K and R residues, linked to plasmin or trypsin activity, is relatively low in the gastric sample. Indeed, the majority of observed cleavages at these residues represent repeated detection of peptides already identified in the intact milk sample, with only 9 new cleavages after K or R residues emerging in the gastric sample. Finally, cleavage after P residues, consistent with the action of proline endopeptidase is also observed at a low frequency in the gastric sample.
Comparing Measures of Enzyme Activity
In human milk, naturally occurring peptides are present at various levels. Therefore, examining only the number of cleavages each enzyme is responsible for may not be representative of true enzyme activity. We thus carried out the same comparison as above by taking into account the abundances of different peptides (Figure 2B). The same overall tendency of enzyme activity, in both the human milk and the gastric sample, is upheld when using both peptide counts and abundance. For intact milk, the highest activity is attributed to plasmin and trypsin, while the lowest is associated with proline endopeptidase. However, there is a slight difference between the two approaches in terms of the inferred activity of elastase and chymotrypsin in intact human milk. Figure 2a, based on cleavage frequency counts, shows that chymotrypsin activity is greater than that of elastase, while the opposite is seen in Figure 2B when abundance is considered. For the gastric sample, we observe consistent results using abundance except for the level of elastase activity compared plasmin/trypsin (Figure 2B versus Figure 2A).
Overall, we see an increase in the observed activity of the enzymes when comparing intact human milk to the gastric sample. Notably, however, according to the peptide counts data, plasmin and trypsin demonstrate greater activity in intact milk than in the gastric sample (Figure 2A), while conversely, the abundance data reports greater activity for plasmin and trypsin in the gastric sample. Currently, it is unclear whether the use of abundance or peptide counts is a more suitable approach, as abundance data is highly dependent on peptide ionization efficiency, which varies between peptides. The use of enzyme activity assays (e.g., Korycha-Dahl et al.23) could serve to address this point and indeed further characterize the enzyme profile of human milk; however, this is beyond the scope of this current study.
Seven enzymes were predicted to be functional in human gastric samples, explaining 432 unique detected cleavages (i.e., N and C cleavages that yield a unique peptide, excluding duplicates based on post-translational modifications). However, approximately 28% (121) of the observed cleavages are not accounted for by the predicted activity of these 7 enzymes. These cleavages may be due to still uncharacterized enzymes or due to additional unknown specificities of the enzymes predicted here. Additionally, this work shows that human milk enzymes continue to function within the infant stomach. Every enzyme predicted in the gastric sample is also predicted to be in human milk (Table 2). The intensity of activity of these enzymes, in terms of number of cleavages performed, however, does vary between the gastric and intact human milk samples.
Temporal Variation in Enzyme Activity through Infant Digestion from Intact Human Milk to Gastric Aspirates
The majority of predicted enzymes demonstrate an increase in activity in the stomach compared with human milk. The notable exceptions are plasmin and trypsin (Table 3; Figure 2A), with their activity experiencing a 23% total decrease in the stomach sample compared to human milk (from 103 cleavages in intact milk to 79 in the gastric sample; Table 3). The greatest amplification in activity in the stomach is observed for chymotrypsin, which exhibits, overall, more than a 2-fold increase over human milk (Table 3). Indeed, the frequency of chymotrypsin cleavage occurring exclusively in the infant stomach (and not as a result of native human milk cleavage) is greater than the total chymotrypsin cleavage observed in human milk (Table 3). Similarly, both pepsin- and cathepsin D-like activity in the gastric sample increase by more than 2-fold over human milk, with the gastric-specific cleavages for both again attaining greater levels to those seen in human milk (Table 3). Finally, a slight increase in proline endopeptidase activity is seen, yielding 9 new gastric-specific cleavages.
Variation in pH Optima among the Predicted Enzymes
To date, very little is known with regard to the gastric enzymes of human neonates. Indeed, the activity of pepsin, the most widely studied of these enzymes, has yet to be identified in infants in the early postpartum period. The prefeed gastric pH of neonates between 2 and 13 days has a mean low of 3.5, which would be suitable for pepsin and cathepsin D activity.5 However, after ingestion of human milk, the pH increases to 5–7 for 2 h.5 The pH optima of gastric detected enzymes are quite variable, for example: maximal pepsin pH is ∼2 (with nearly 40% of activity at pH 524), maximal cathepsin D pH is 3.5 (with over 25% of activity at pH 525), maximal chymotrypsin activity occurs between pH 6.5 and 7.5 (with lesser activity to at least pH 5.526) and maximal elastase activity occurs at pH 8.5 (with lesser activity to at least pH 5.527). Our data suggest that despite the suboptimal conditions for these enzymes in the infant stomach, their activity is maintained.
Conclusion
The digestive process in infants remains largely unstudied. However, based on previous data demonstrating a pH of 5–7 post milk ingestion, the accepted dogma has been that little to no pepsin-induced digestion occurs in the infant stomach for at least 13 days postpartum.7 From our present study, following the digestion of human milk in 4–12 day postpartum infants, we propose a new paradigm for digestion of milk proteins and peptides in the first 2 weeks of life: (1) numerous indigenous milk peptides remain intact throughout digestion (Figure 1A), (2) some indigenous milk peptides can be subject to further cleavage (Figure 1B), and (3) some novel milk peptides are released upon ingestion (Figure 1C).
While the sample size of this current study is undoubtedly limited, we do present important observations pertaining to human milk digestion that merit further investigation. Of the 7 enzymes predicted to be active in human milk and in gastric samples, 5 show increased activity in the stomach. For example, an over 2-fold increase is seen for chymotrypsin-, pepsin-, and cathepsin D-like activity. Some of this increased activity can be explained by the slightly lower pH of the infant stomach postfeeding in comparison to that of human milk. However, it is harder to explain our observation of increased elastase-like activity in the stomach, as its optimal pH is more basic. Surprisingly, we find that the activity of plasmin and/or trypsin continues in the stomach, but decreases compared to the observed activity in intact human milk. Finally, since our analysis fails to account for the activity of approximately 22% of the detected cleavages specific to the infant stomach, it is clear that there is still much to learn about human milk and digestion in the neonate. This study provides a cursory outline of the enzymatic activities in human milk but undoubtedly there is more to discover. Future experimental work is required to fully characterize the enzyme profile of both human milk and the gastric environment of the neonate, and indeed to determine the origin of the novel cleavage activities detected in our analysis.
This work was funded by the Irish Research Council for Science, Engineering and Technology, co-funded by Marie Curie Actions under FP7 (N. Khaldi); Enterprise Ireland grant (CC20080001) to Food for Health Ireland; Science Foundation Ireland grant 08/IN.1/B1864; University of California Discovery Program (05GEB01NHB); National Science Foundation Graduate Research Fellowship Program (D.C. Dallas) and the USDA National Institute for Food and Agriculture Post-doctoral Fellowship (D.C. Dallas), the National Institutes of Health (R01 HD059127 and UL1 TR000002).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Dallas D. C.; Guerrero A.; Khaldi N.; Castillo P. A.; Martin W. F.; Smilowitz J. T.; Bevins C. L.; Barile D.; German J. B.; Lebrilla C. B. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J. Proteome Res. 2013, 1252295–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldi N.; Vijayakumar V.; Dallas D. C.; Guerrero A.; Wickramasinghe S.; Smilowitz J. T.; Medrano J. F.; Lebrilla C. B.; Shields D. C.; German J. B. Predicting the important enzyme players in human breast milk digestion. J. Agric. Food Chem. 2014, 62297225–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene M.; Hewer E. Digestive enzymes of the human foetus. Lancet 1929, 2135511767–769. [Google Scholar]
- Defize J.; Meuwissen S. Pepsinogens: An update of biochemical, physiological, and clinical aspects. J. Pediatr. Gastroenterol. Nutr. 1987, 64493–508. [PubMed] [Google Scholar]
- Mason S. Some aspects of gastric function in the newborn. Arch. Dis. Child. 1962, 37194387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand M.; Hamosh M.; Mehta N. R.; Angelus P. A.; Philpott J. R.; Henderson T. R.; Dwyer N. K.; Lairon D.; Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr. Res. 1996, 403429–437. [DOI] [PubMed] [Google Scholar]
- Dallas D. C.; Underwood M. A.; Zivkovic A. M.; German J. B. Digestion of protein in premature and term infants. J. Nutr. Disord. Ther. 2012, 21121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silanikove N.; Merin U.; Leitner G. Physiological role of indigenous milk enzymes: An overview of an evolving picture. Int. Dairy J. 2006, 166533–545. [Google Scholar]
- Jensen R. G.Handbook of Milk Composition; Academic Press: San Diego, CA, 1995; access online at http://www.sciencedirect.com/science/book/9780123844309. [Google Scholar]
- Shahani K.; Kwan A.; Friend B. A. Role and significance of enzymes in human milk. Am. J. Clin. Nutr. 1980, 3381861–1868. [DOI] [PubMed] [Google Scholar]
- Dallas D. C.; Guerrero A.; Khaldi N.; Borghese R.; Bhandari A.; Underwood M. A.; Lebrilla C. B.; German J. B.; Barile D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J. Nutr. 2014, 1446815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas D. C.; Martin W. F.; Strum J. S.; Zivkovic A. M.; Smilowitz J. T.; Underwood M. A.; Affolter M.; Lebrilla C. B.; German J. B. N-Linked glycan profiling of mature human milk by high-performance microfluidic chip liquid chromatography time-of-flight tandem mass spectrometry. J. Agric. Food Chem. 2011, 5984255–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferranti P.; Traisci M. V.; Picariello G.; Nasi A.; Boschi V.; Siervo M.; Falconi C.; Chianese L.; Addeo F. Casein proteolysis in human milk: Tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J. Dairy Res. 2004, 710174–87. [DOI] [PubMed] [Google Scholar]
- Craig R.; Beavis R. C. TANDEM: Matching proteins with tandem mass spectra. Bioinformatics 2004, 2091466–1467. [DOI] [PubMed] [Google Scholar]
- Molinari C. E.; Casadio Y. S.; Hartmann B. T.; Livk A.; Bringans S.; Arthur P. G.; Hartmann P. E. Proteome mapping of human skim milk proteins in term and preterm milk. J. Proteome Res. 2012, 1131696–1714. [DOI] [PubMed] [Google Scholar]
- Mangé A.; Bellet V.; Tuaillon E.; Van de Perre P.; Solassol J. Comprehensive proteomic analysis of the human milk proteome: Contribution of protein fractionation. J. Chromatogr., B 2008, 8762252–256. [DOI] [PubMed] [Google Scholar]
- Liao Y.; Alvarado R.; Phinney B.; Lönnerdal B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 2011, 1083530–3541. [DOI] [PubMed] [Google Scholar]
- Vijayakumar V.; Guerrero A.; Davey N.; Lebrilla C. B.; Shields D. C.; Khaldi N. EnzymePredictor: A tool for predicting and visualizing enzymatic cleavages of digested proteins. J. Proteome Res. 2012, 11126056–6065. [DOI] [PubMed] [Google Scholar]
- Considine T.; Healy Á.; Kelly A. L.; McSweeney P. L. H. Hydrolysis of bovine caseins by cathepsin B, a cysteine proteinase indigenous to milk. Int. Dairy J. 2004, 142117–124. [Google Scholar]
- Considine T.; Geary S.; Kelly A. L.; McSweeney P. L. H. Proteolytic specificity of cathepsin G on bovine αs1- and β-caseins. Food Chem. 2002, 76159–67. [Google Scholar]
- Kelly A. L.; O’Flaherty F.; Fox P. F. Indigenous proteolytic enzymes in milk: A brief overview of the present state of knowledge. Int. Dairy J. 2006, 166563–572. [Google Scholar]
- Raymond W. W.; Trivedi N. N.; Makarova A.; Ray M.; Craik C. S.; Caughey G. H. How immune peptidases change specificity: Cathepsin G gained tryptic function but lost efficiency during primate evolution. J. Immunol. 2010, 18595360–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korycha-Dahl M.; Dumas B. R.; Chene N.; Martal J. Plasmin activity in milk. J. Dairy Sci. 1983, 664704–711. [Google Scholar]
- Piper D.; Fenton B. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 65506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowska-Jakimiec W.; Dabrowska E.; Gacko M.; Karwowska A.; Chlabicz M. The choice of conditions for cathepsin D activity determination in human saliva. Adv. Med. Sci. 2006, 51S1179–181. [PubMed] [Google Scholar]
- Schwert G. W.; Takenaka Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim. Biophys. Acta 1955, 16, 570–575. [DOI] [PubMed] [Google Scholar]
- Ohlsson K.; Odsson I. The neutral proteases of human granulocytes. Eur. J. Biochem. 1974, 422519–527. [DOI] [PubMed] [Google Scholar]