Abstract
A three-step route was used to synthesize 1,7-bis(t-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (DO2A-t-Bu ester) from 1,4,7,10-tetraazacyclododecane (cyclen). The overall time of reaction was reduced from a combined ~56 h to 2.3 h with an overall yield comparable to previously reported methods.
Keywords: DO2A-t-Bu Ester, cyclen, transfer hydrogenation, microwave
Derivatives of 1,4,7,10-tetraazacyclododecane (cyclen), including 1,7-bis(t-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (DO2A-t-Bu ester, 1), are commonly used intermediates when synthesizing metal complexes for biomedical-related studies.1–19 However, reported routes to synthesize and purify this intermediate require multiple days to complete.20–22 We hypothesized that the reactions used to produce 1 could be accelerated by modification of the reaction conditions, leading to a shortened overall synthetic time while maintaining comparable yields to previously reported reactions.
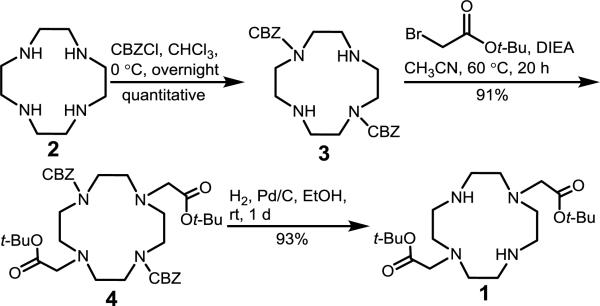
The reported synthesis of DO2A-t-Bu ester, 1, is carried out in three steps as depicted in Scheme 1.20–22 First, two amines of commercially available cyclen are protected in the trans positions with a slow addition of CBZCl at 0 °C and stirring overnight.20 Second, t-Bu bromoacetate is reacted with CBZ-protected cyclen, 3, at 60 °C in the presence of diisopropylethylamine (DIEA) to produce macrocycle 4 in 20 h.21 Finally, hydrogenation for 1 d removes the CBZ groups yielding the desired product 1.21
Scheme 1.
Reported synthesis of DO2A-t-Bu ester, 1, with shortest reported times shown.20–22
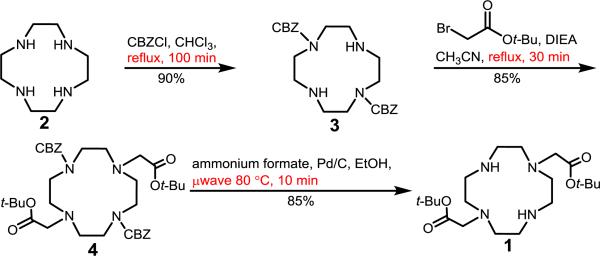
We observed that the reaction time from 2 to 3 can be reduced with the application of heat, leading to a decrease in reaction time from 12 h to 30 min at reflux (Scheme 2). Temperatures below reflux led to either longer reaction times or lower yields. We verified that heating did not produce the undesired cis-substituted product by comparing our final product, 1, with a reported NMR spectrum of 1,4-bis(t-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane.23 Based upon our observations, heating the first step at reflux leads to the exclusive formation of the trans-substituted intermediate 3.
Scheme 2.
Modified synthesis of 1 with modifications shown in red.
The reported reaction of 3 to 4 requires heating at 60 °C with 2 equiv of base for 20 h to reach completion. We predicted that the addition of excess base would allow for a shorter overall reaction time because of the similar pKa values of DIEA and the amines on macrocycle 3 (all in the range of 9–10). Consequently, extra base should minimize protonation of the amines on 3, allowing the desired reaction to occur. At 60 °C with a 20-fold excess of base per equivalent of 3, this reaction took 50 min to reach completion compared to 20 h with 2 equiv of base, demonstrating that the amount of base is a key component of the reaction. To further accelerate the reaction, we heated at reflux instead of 60 °C, leading to a total reaction time of 30 min with 85% isolated yield (Scheme 2).
The final step from CBZ-protected 4 to product 1 was accelerated by transfer hydrogenation with ammonium formate and microwave irradiation. Ammonium formate is a more user-friendly reagent than H2 gas, and similar microwave-assisted transfer hydrogenation reactions have been performed to deprotect CBZ-protected amines.24 With the addition of ammonium formate under microwave irradiation in a sealed vessel, the reaction was complete after 10 min at 80 °C. An excess of ammonium formate was used because some sublimation was observed during the reaction, and heating above 80 °C increased the amount of sublimation that was observed. This third reaction was complete in 10 min, compared to the reported route that requires a reaction time of 1 d at room temperature with H2 gas.
Due to the decreases in reaction times for all three steps, it is possible to synthesize DO2A-t-Bu ester, 1, from commercially available cyclen in one day. We have performed the reactions leading to 1 starting from up to 5 g of cyclen with comparable yields to smaller scales, and we expect that even larger scale reactions would behave similarly.25 Furthermore, similar yields to DO2A-t-Bu ester were obtained for DO2A-methyl ester and DO2A-ethyl ester. The ability to rapidly synthesize methyl, ethyl, and t-Bu ester variants of DO2A has the potential to greatly aid studies that use these molecules as intermediates.
Supplementary Material
Acknowledgments
The authors acknowledge the Lumigen Instrument Center at Wayne State University. L.E.H. thanks the National Institutes of Health (GM 058905) for support. M.J.A. acknowledges the National Science Foundation (CHE-0955000).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/. These data include detailed experimental procedures and 1H- and 13C-NMR spectra.
References and notes
- 1.Vibhute SM, Engelmann J, Verbić T, Maier ME, Logothetis NK, Angelovski G. Org. Biomol. Chem. 2013;11:1294–1305. doi: 10.1039/c2ob26555a. [DOI] [PubMed] [Google Scholar]
- 2.Szíjjártó C, Pershagen E, Borbas KE. Dalton Trans. 2012;41:7660–7669. doi: 10.1039/c2dt30569k. [DOI] [PubMed] [Google Scholar]
- 3.Polasek M, Caravan P. Inorg. Chem. 2013;52:4084–4096. doi: 10.1021/ic400227k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que EL, New EJ, Chang CJ. Chem. Sci. 2012;3:1829–1834. doi: 10.1039/C2SC20273E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty D, Jeong JM, Ju CH, Lee Y-S, Jeong SY, Choi JY, Yang BY, Lee DS, Chung J-K, Lee MC. Nucl. Med. Bio. 2010;37:893–902. doi: 10.1016/j.nucmedbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Abiraj K, Jaccard H, Kretzschmar M, Helm L, Maecke HR. Chem. Commun. 2008:3248–3250. doi: 10.1039/b805281f. [DOI] [PubMed] [Google Scholar]
- 7.Harvey P, Chalmers KH, De Luca E, Mishra A, Parker D. Chem. Eur. J. 2012;18:8748–8757. doi: 10.1002/chem.201200737. [DOI] [PubMed] [Google Scholar]
- 8.Pal R, Parker D. Org. Biomol. Chem. 2008;6:1020–1033. doi: 10.1039/b718993a. [DOI] [PubMed] [Google Scholar]
- 9.Keizers PHJ, Saragliadis A, Hiruma Y, Overhand M, Ubbink M. J. Am. Chem. Soc. 2008;130:14802–14812. doi: 10.1021/ja8054832. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A, Mishra R, Gottschalk S, Pal R, Sim N, Engelmann J, Goldberg M, Parker D. ACS Chem. Neurosci. 2014;5:128–137. doi: 10.1021/cn400175m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson KL, Margherio MJ, Doan P, Wilke KT, Pierre VC. Inorg. Chem. 2013;52:9390–9398. doi: 10.1021/ic4009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchardt C, Riss PJ, Zoller F, Maschauer S, Prante O, Kuwert T, Roesch F. Bioorg. Med. Chem. Lett. 2009;19:3498–3501. doi: 10.1016/j.bmcl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Riss PJ, Burchardt C, Zimny MJ, Peters J, Roesch F. RSC Adv. 2012;2:7156–7160. [Google Scholar]
- 14.Angelovski G, Chauvin T, Pohmann R, Logothetis NK, Tóth É . Bioorg. Med. Chem. 2011;19:1097–1105. doi: 10.1016/j.bmc.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Keizers PHJ, Desreux JF, Overhand M, Ubbink MJ. Am. Chem. Soc. 2007;129:9292–9293. doi: 10.1021/ja0725201. [DOI] [PubMed] [Google Scholar]
- 16.Law G-L, Man C, Parker D, Walton JW. Chem. Commun. 2010;46:2391–2393. doi: 10.1039/b924679g. [DOI] [PubMed] [Google Scholar]
- 17.Law G-L, Pal R, Palsson LO, Parker D, Wong K-L. Chem. Commun. 2009:7321–7323. doi: 10.1039/b920222f. [DOI] [PubMed] [Google Scholar]
- 18.Parker D, Walton JW, Lamarque L, Zwier JM. Eur. J. Inorg. Chem. 2010:3961–3966. [Google Scholar]
- 19.Hirayama T, Taki M, Kodan A, Kato H, Yamamoto Y. Chem. Commun. 2009:3196–3198. doi: 10.1039/b900302a. [DOI] [PubMed] [Google Scholar]
- 20.De León-Rodríguez LM, Kovacs Z, Esqueda-Oliva AC, Miranda-Olvera AD. Tetrahedron Lett. 2006;47:6937–6940. [Google Scholar]
- 21.Kovacs Z, Sherry AD. J. Chem. Soc., Chem. Commun. 1995:185–186. [Google Scholar]
- 22.Kovacs Z, Sherry AD. Synthesis. 1997:759–763. [Google Scholar]
- 23.Li C, Wong W-T. J. Org. Chem. 2003;68:2956–2959. doi: 10.1021/jo026436+. [DOI] [PubMed] [Google Scholar]
- 24.Daga MC, Taddei M, Varchi G. Tetrahedron Lett. 2001;42:5191–5194. [Google Scholar]
- 25.The scaled up microwave reaction was heated in an open vessel connected to a condenser located outside of the microwave cavity instead of a sealed vessel.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.